Book:The Comprehensive Guide to Physician Office Laboratory Setup and Operation/The clinical environment/Data management
1.10 Data management
The role of the laboratory is shifting both by internal forces, like the adoption of new technology, and external ones, such as consumerism, information availability, and the development of complicated data streams. Laboratory professionals are moving to a role that places them in the center of the data stream. As such, effective data collection and management is becoming more important than ever. This requires not only quality tools but also smooth, consistent workflows that can easily be followed and updated.
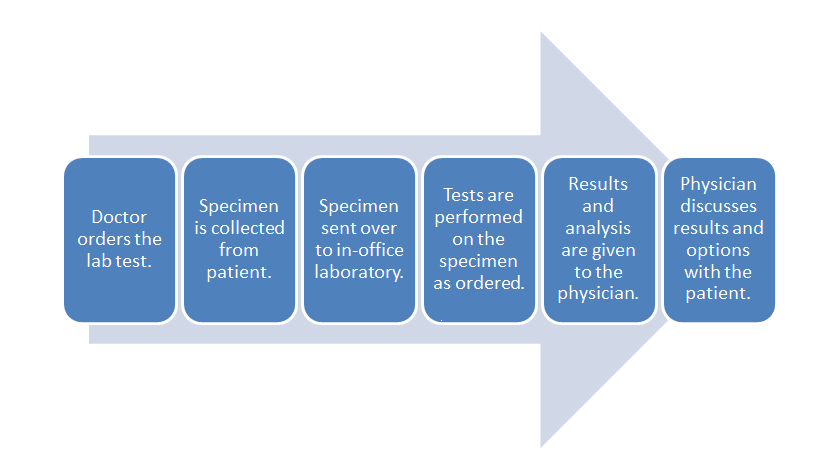
- POL workflow:
The above diagram shows the typical workflow for the POL. While managing this workflow is important, the data associated with it goes beyond ordinary practice function; the data can be used to assist with tracking population health for the patients or be combined with financial data such as billing or spending on supplies.
Other data streams may be generated outside this typical workflow as well. For example, the enactment of Meaningful Use Stage 3 by 2018 (the program was renamed from "Meaningful Use" to "Promoting Interoperability"[1][2]) meant EHRs had to be able to accept patient-generated data. This meant data coming from tracking tools like Fitbits or glucometers used by the patient had to be able to be accepted into an EHR.[3] This data, along with data from other POCT devices, aid the physician with diagnosis by giving them more data points to examine; however, this also requires the practice to have strong data management methods.
This area is a future frontier in the quest to use technology as a means of improving outcomes while driving costs down. The popularity of these patient devices and the trend towards using technology for healthcare purposes is drawing physician interest in using such devices for expanded monitoring of patients, with indicators supporting this trend on the development side.[3][4] The previously mentioned Healthy.io and its urinalysis strip than can be analyzed with a mobile app serves as one example.[5][6] Results can be stored and interpreted via the app, and that data can eventually make its way into a physician's compliant EHR. However, as Dihn-Le et al. pointed out in 2019, "[i]ncreased partnerships and opportunities between makers of these applications and health systems are necessary to reach high interoperability and streamlined communication between EHR platforms, patient devices, and providers."[4]
1.10.1 Data management tools and challenges
Laboratory data management for a POL has largely been done manually on paper or using office software like Excel. As technology has progressed, some of the larger physician office groups have adopted more sophisticated tools like the LIS, a software system that records, manages, and stores data for clinical laboratories. The LIS is able to connect to laboratory instruments, track orders, and record and store results. A modern LIS is also capable of integrating with an EHR to allow the results to be stored in the complete patient record. However, a full-featured LIS may be too much software for smaller POLs. Some companies like Relaymed have tried to solve this challenge by introducing cloud-based middleware-like solutions that help integrate POCT instruments and wearables with the EHR and test orders to make smoother POC workflows and data management in the POL.[7]
Other tools for connecting the lab with the physician office have emerged. Clinical information exchange (or health information exchange) systems have been designed to optimize workflows using application programming interfaces (APIs) and web services technology to connect systems.[8] (In fact, the previously mentioned Relaymed system is arguably in many ways a clinical information exchange system.) Computerized provider order entry (CPOE) systems are also increasingly being used. In many cases these systems are used for entering drug orders, but these systems can also be used to enter laboratory testing orders. This offers an opportunity for laboratory professionals to assist physicians with choosing the appropriate test.[9]
When done well, laboratory data management can allow physician offices to track data for multiple business processes as well as patient care. This is made easier when software systems and POCT instruments are integrated and the staff is trained in their use. As such, laboratory personnel should be part of the data management system decision making processes, especially given the introduction of patient-generated laboratory data. This also provides an opportunity for laboratory directors to get involved, as they are the ones ultimately responsible for compliance with regulatory issues, particularly since CLIA regulations hold laboratory directors responsible for compliance with data-reporting EHR requirements. Yes, the POL is not impacted by some of the challenges regarding CLIA requirements; however, it can still be impacted by the requirements to collect data from multiple sources and put that data into the patient record.[10] As most POLs are operating on a CLIA certificate of waiver and the requirements for the laboratory director are minimal, an air of complacency can evolve in the POL environment. However, a POL's laboratory director must focus on being proactively involved with data collection and management functions, as well as the EHR and LIS selection and familiarization process.
References
- ↑ Bresnick, J. (24 April 2018). "CMS Renames Meaningful Use to Highlight Interoperability Goals". Health IT Analytics. https://healthitanalytics.com/news/cms-renames-meaningful-use-to-highlight-interoperability-goals. Retrieved 21 April 2022.
- ↑ AAP Division of Quality (13 June 2019). "Meaningful use program renamed; stage 3 requirements revised". AAP News. American Academy of Pediatrics. https://publications.aap.org/aapnews/news/11909. Retrieved 21 April 2022.
- ↑ 3.0 3.1 McLeod, Pamela Scherer (19 March 2015). "Physicians Use Fitness Trackers to Monitor Patients in Real-time, Even as Developers Work to Incorporate Medical Laboratory Tests into the Devices". Dark Daily. Dark Intelligence Group, Inc. http://www.darkdaily.com/physicians-use-fitness-trackers-to-monitor-patients-in-real-time-even-as-developers-work-to-incorporate-medical-laboratory-tests-into-the-devices-528. Retrieved 14 April 2015.
- ↑ 4.0 4.1 Dinh-Le, Catherine; Chuang, Rachel; Chokshi, Sara; Mann, Devin (2019). "Wearable Health Technology and Electronic Health Record Integration: Scoping Review and Future Directions". JMIR mHealth and uHealth 7 (9). doi:10.2196/12861. ISSN 2291-5222. PMC 6746089. PMID 31512582. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6746089/.
- ↑ Buhr, Sarah (18 February 2015). "Scanadu’s New Pee Stick Puts The Medical Lab On Your Smartphone". Tech Crunch. Yahoo. https://techcrunch.com/2015/02/18/scanadus-new-pee-stick-puts-the-medical-lab-on-your-smartphone/. Retrieved 20 April 2022.
- ↑ "About Us". Healthy.io. https://healthy.io/about-us. Retrieved 20 April 2022.
- ↑ "An alternative to a Lab Information System (LIS)". Relaymed Blog. Relaymed. 30 November 2021. https://relaymed.com/blog/an-alternative-to-lab-information-system/. Retrieved 21 April 2022.
- ↑ Michel, Robert (30 March 2015). "How Medical Laboratories Help Physicians Overcome the Failure of Many EHR Systems to Support Effective Lab Test Ordering and Lab Result Reporting". DarkDaily.com. Dark Intelligence Group, Inc. http://www.darkdaily.com/how-medical-laboratories-help-physicians-overcome-the-failure-of-many-ehr-systems-to-support-effective-lab-test-ordering-and-lab-result-reporting-330.
- ↑ Sinard, John (2006). Practical Pathology Informatics: Demystifying Informatics for the Practicing Anatomic Pathologist. Springer Science & Business Media. pp. 412. ISBN 9780387280585. https://books.google.com/books?id=WerUyK618fcC. Retrieved 21 April 2022.
- ↑ Henricks, Walter H. (18 March 2015). "Accreditation and Regulatory Implications of Electronic Health Records for Laboratory Reporting". InsuranceNewsNet.com, Inc. Archived from the original on 07 August 2015. http://insurancenewsnet.com/oarticle/2015/03/18/accreditation-and-regulatory-implications-of-electronic-health-records-for-labor-a-606123.html. Retrieved 21 April 2022.
Citation information for this chapter
Chapter: 1. The clinical environment
Title: The Comprehensive Guide to Physician Office Laboratory Setup and Operation
Edition: Second edition
Author for citation: Shawn E. Douglas
License for content: Creative Commons Attribution-ShareAlike 4.0 International
Publication date: June 2022