Journal:A high-throughput method for the comprehensive analysis of terpenes and terpenoids in medicinal cannabis biomass
| Full article title | A high-throughput method for the comprehensive analysis of terpenes and terpenoids in medicinal cannabis biomass |
|---|---|
| Journal | Metabolites |
| Author(s) | Krill, Christian; Rochfort, Simone; Spangenberg, German |
| Author affiliation(s) | AgriBio, the Centre For AgriBioscience |
| Primary contact | Email: christian dot krill at agriculture dot vic dot gov dot au |
| Year published | 2020 |
| Volume and issue | 10(7) |
| Article # | 276 |
| DOI | 10.3390/metabo10070276 |
| ISSN | 2218-1989 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.mdpi.com/2218-1989/10/7/276/htm |
| Download | https://www.mdpi.com/2218-1989/10/7/276/pdf (PDF) |
|
|
This article contains rendered mathematical formulae. You may require the TeX All the Things plugin for Chrome or the Native MathML add-on and fonts for Firefox if they don't render properly for you. |
Abstract
Cannabis and its secondary metabolite content have recently seen a surge in research interest. Cannabis terpenes and terpenoids in particular are increasingly the focus of research efforts due to the possibility of their contribution to the overall therapeutic effect of medicinal cannabis. Current methodology to quantify terpenes in cannabis biomass mostly relies on large quantities of biomass, long extraction protocols, and long gas chromatography (GC) gradient times, often exceeding 60 minutes. They are therefore not easily applicable in the high-throughput environment of a cannabis breeding program. The method presented here, however, is based on a simple hexane extract from 40 mg of biomass, with 50 μg/mL dodecane as internal standard, and a gradient of less than 30 minutes. The method can detect 48 individual terpenes and terpenoids and was validated for selectivity, linearity, limit of detection/limit of quantitation (LOD/LOQ), precision, intermediate precision, and accuracy (recovery) for 22 terpenes and terpenoids. The validation parameters are comparable to previously published studies that employ significantly longer runtimes and/or more complex extraction protocols. It is currently being applied to medicinal cannabis precision breeding programs.
Keywords: cannabis volatiles, terpenes, terpenoids, profiling, high-throughput method
Introduction
Cannabis is a genus of annual dioecious plants within the family Cannabaceae with a rich and complex constituency of pharmacologically relevant phytochemicals.[1][2] Under the Single Convention on Narcotic Drugs (1961), cannabis was deemed to be a plant without medicinal purpose, a conclusion based on very little scientific evidence or clinical trial data.[3] As a result, medicinal use of cannabis was practically prohibited by its status as an illegal narcotic worldwide, and research into cannabis chemistry and biology was largely limited to law enforcement and forensics applications.[4] Changes in the attitudes towards cannabis across various societies and increasing evidence for the benefits of cannabinoids in the treatment of otherwise intractable conditions have precipitated recent changes to legislature in a number of jurisdictions, including Australia.[3] Interest in the medicinal uses of cannabis is increasing, as indicated by a growing number of review articles on the topic.[5][6][7][8]
Cannabis, cannabis extracts, and purified (to varying degrees) major cannabinoids such as Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are now being used for the treatment of intractable and debilitating illnesses, such as Dravet’s Syndrome, and investigated for a range of others.[5][7][9][10][11][12] When administered as medicinal cannabis extracts, that is, along with the remaining portion of other cannabinoids and non-cannabinoid phytochemicals, less than a quarter of the equivalent dose of purified CBD is required, and markedly lower side effects are commonly observed.[7] This is in-line with a long line of anecdotal evidence that complex plant-based medicines are more effective than their isolated “actives,” which has given rise to the concept of the “entourage effect,” the modulating or synergistic effects of phytochemical compounds such as the minor cannabinoids on those effects related to THC.[13][14][15] Despite a current lack of understanding for the underlying mechanisms[16], the concept is increasingly applied to non-cannabinoid cannabis phytochemicals, such as terpenes and terpenoids.[6][9][10][17]
Terpenes are a class of hydrocarbons synthesized from the five-carbon (C5) “building blocks” dimethylallyl pyrophosphate and isopentenyl pyrophosphate. Depending on the number of C5 units and possible substitutions, they are further classified based on number of units (e.g., C10 monoterpenes [two sub-units], C15 sesquiterpenes [three sub-units]) or functional groups (terpenoids and oxygenated). Mono- and sesquiterpenes are classified as volatile and semi-volatile compounds, respectively, and higher order terpenes (e.g., C20 diterpenes and C30 triterpenes) exist as steroids, waxes, and resins. Cannabis mono- and sesquiterpenes are responsible for the characteristic smell of the plant and its products. They are also present at pharmacologically relevant concentrations[15][17][18][19], and a growing body of evidence suggests they have pharmacological properties in their own rights.[20][21] Their common use as food additives and in cosmetics products highlights the inherent safety of these compounds.[20]
As Leghissa and colleagues note[22], conclusively identifying terpenes in cannabis is challenging due to the large variety of possible candidates and a lack of commercial standards for a large number of them. With the increased interest in medicinal cannabis and the contribution of terpenes to the entourage effect, methods to quantify terpenes in medicinal cannabis biomass and products are becoming more available in the literature.[23][24][25][26] Often, these methods require large quantities of biomass (1–5 g), use large quantities of organic solvents (up to 100 mL/sample), and include separations with gradient runtimes exceeding 60 minutes. These methods are therefore not easily applied in a high-throughput manner. To facilitate terpene quantitation and profiling in order to enable accelerated precision breeding programs in medicinal cannabis, we developed a fast (30-minute separation), microscale (40 mg sample), high-throughput gas chromatography–mass spectrometry (GC-MS) terpene profiling and quantitation method.
Results
Sampling techniques
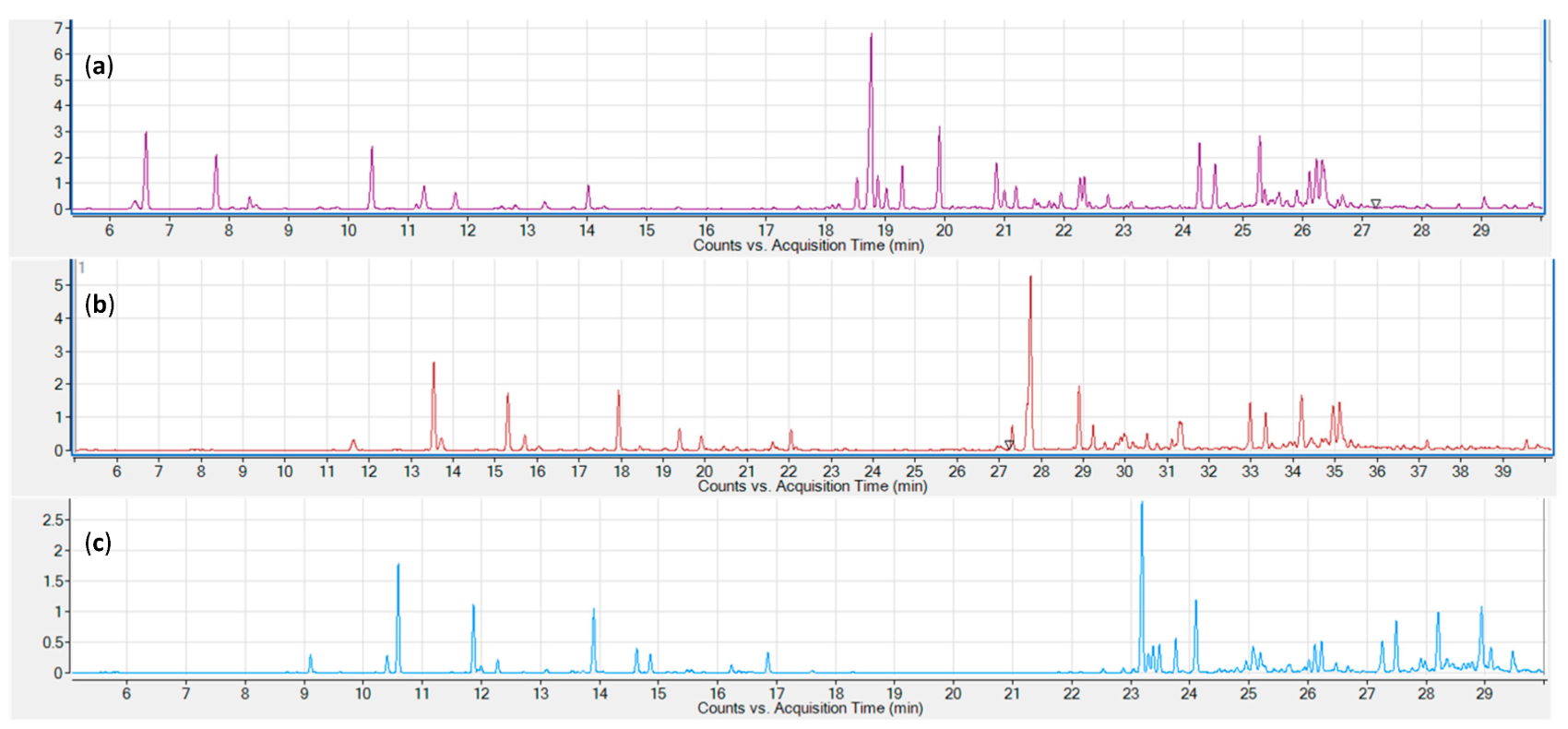
Three common sampling and sample introduction techniques were evaluated: static headspace, solid-phase microextraction (SPME), and liquid injection of an organic solvent extract. Representative chromatograms for the three tested sampling techniques are shown in Figure 1. All three techniques provide excellent signal strengths for the lower boiling monoterpenes. Sesquiterpenes are underrepresented in the static headspace chromatogram (a), while the SPME chromatogram shows a stronger signal for early eluting sesquiterpenes. Higher boiling sesquiterpenes are only adequately represented in the hexane extract chromatogram.
|
Columns
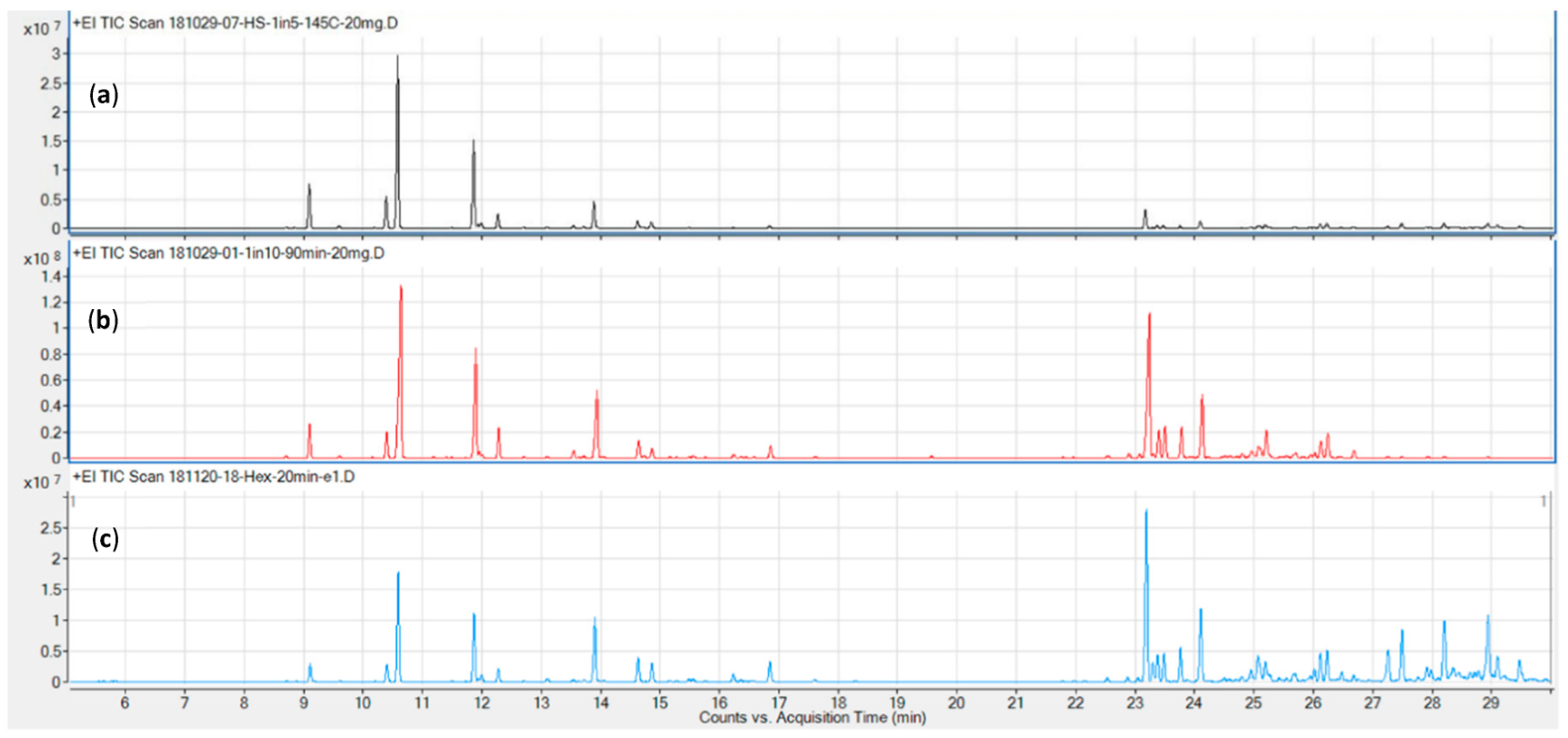
Representative chromatograms for the three columns tested (DB5, DB17, and VF35, in order of increasing polarity) are shown in Figure 2. The DB5 (c) and DB17 (a) columns performed comparably well in terms of resolution and overall run time (≤ 30 minutes), while the VF 35 column required a runtime in excess of 35 minutes for the chosen temperature program.
|
Solvent optimisation
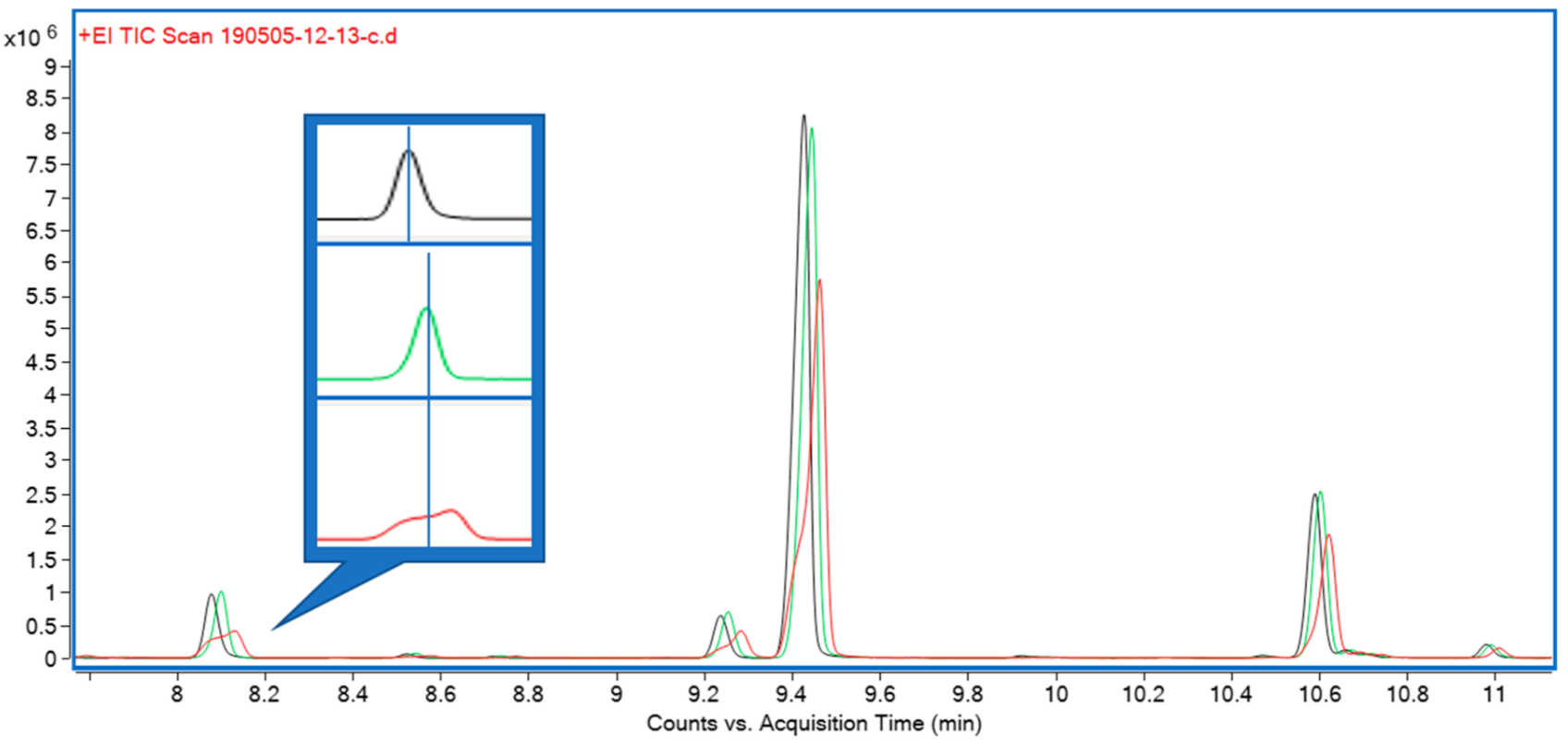
Three solvents—hexane, isopropanol, and ethyl acetate—were initially trialed for liquid extraction. All three performed equally well in terms of extraction efficiency, but both isopropanol and, to a lesser extent, ethyl acetate were causing early eluting compounds to show undesirable peak shapes during gas chromatography (GC) analysis. A comparison of peak shapes is shown in Figure 3. Furthermore, hexane extracts appeared as light green, clear extracts, whereas ethyl acetate and isopropanol extracts were opaque and of a much darker color, indicating the presence of a higher proportion of non-target compounds.
|
Compound identification and resolution
A total of 48 individual compounds were detected across multiple strains used. Of those, 22 were monoterpenes and -terpenoids, with the remainder consisting of sesquiterpenes and -terpenoids. Compound names, base peak (quantifier) ion m/z, retention times and indices, and identification status are summarized in Table 1. Most monoterpene identities predicted by spectral library matching were confirmed using authentic standards, with the exception of fenchol and trans-2-pinanol. The α thujene, β-phellandrene, and thujanol identities were confirmed by matching published retention times indices (RIs). Sesquiterpene preliminary identities were confirmed using authentic standards for eight out of 25 compounds (Table 1), and RI matching for a further three.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Resolution was calculated based on the following equation (discussed further in the "Materials and methods" section):
Values presented in Table 1 are based on the respective base peak (qualifier) ion and are given for the closest preceding (Rp) and following peak (Rs).
Linearity
A combined cannabis terpenes (CT) and sabinene standard was injected at ten concentrations from 100 μg/mL to 0.195 ng/mL, based on a 1:2 dilution series of the 100 μg/mL standard. All calibration curves obtained were linear with R2 values greater than or equal to 0.994. Based on individual detection limits, the curves spanned all ten calibration points, except linalool, ocimene, and trans-caryophyllene (100–0.391 μg/mL); caryophyllene oxide, guaiol, and isopulegol (100–1.56 μg/mL); and α-bisabolol and β-cis-caryophyllene (100–12.5 μg/mL). Nerolidol was removed from the analysis due to poor peak shapes and high detection limits.
Detection and quantitation limits
Limit of detection (LOD) and limite of quantitation (LOQ) were determined as LOD = 3.3 × σ/S and LOQ = 10 × σ/S, with σ representing the standard deviation of the compound and S the slope of the compound’s calibration curve. Detection limits range from 0.017 μg/mL to 1.144 μg/mL (Table 2); quantitation limits range from 0.052 to 3.468 μg/mL (β-pinene and α-bisabolol for both LOD/LOQ).
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Accuracy
To determine method accuracy, three replicates of a sample preparation were spiked with 0.5, 0.25, and 0.05 mg/g of CT and sabinene and extracted as described above. Unspiked biomass was extracted to determine endogenous levels. The recovery requirements of the spike as expressed in % adjusted for endogenous levels were 80–120%. Measured values ranged from 61.8% to 121.4%, with the majority of compounds ranging from 80% to 120% recovery across the three concentrations (Table 3).
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Precision and intermediate precision
Eight individual preparations of biomass were extracted independently by two analysts on different days and analyzed separately. Relative standard variations (%RSD) varied from 1.13 to 8.74 for single analyses, 1.65 to 7.54 for both analyses combined (Table 4). Three compounds—α-terpinene, eucalyptol, and terpinolene—were found to have %RSDs > 10. Results are summarized in Table 4. Myrcene content was determined from a 1:5 dilution of the original extract to bring the concentration back into the linear range.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Discussion
In recent years, the push from society to employ medicinal cannabis in the treatment of intractable and debilitating illnesses has been met by some governments legalizing research into medicinal cannabis.[3][4][5] Research in this area has escalated, but the understanding of the complex composition of cannabis extract is still rudimentary.[16] In one such program, we seek to profile and measure terpenes and terpenoids in medicinal cannabis inflorescence through GC-MS to inform genome-wide association studies (GWAS) and other aspects of medicinal cannabis accelerated precision breeding for the development of novel designer strains. The method should be:
- comprehensive, detecting the highest possible number of individual compounds;
- quantitative, reliably providing absolute quantitation for as many compounds as possible; and
- high-throughput, processing the maximum number of samples possible in as short a time as possible, from as little biomass as possible.
For our purposes, absolute quantitation, while desirable, is not strictly a prerequisite, as meaningful correlations to genomics data can be drawn from relative quantities, i.e., analyte-internal standard response ratios. Similarly, baseline separation, defined as R > 1.5 and generally a requirement for validated methods, is not our priority. As we are expecting close to 1000 offspring from two to three individual crossing events, resulting in 5,000 potential samples (including replication), increasing sample throughput over existing methods was a major consideration.
We evaluated a range of sample extraction and introduction strategies, two headspace techniques (static headspace and solid-phase microextraction [SPME]), and solvent extraction. SPME was evaluated for its ease of use and comparably high sensitivity.[27] It has been used extensively for volatile profiling in cannabis and cannabis products[28][29], and has been reviewed[22]; however, to our knowledge, none of these methods were validated for quantitation. Most of our preliminary identification work was carried out using SPME, and the method reliably provided very strong signals for all monoterpenes and earlier eluting sesquiterpenes. Higher boiling sesquiterpenes were present, but their signals were comparatively weaker, most likely due to insufficient volatility at the extraction temperature or insufficient fiber incubation. Higher extraction temperatures (> 100 °C) may aid in volatilizing these higher boiling compounds, but this will most likely result in a loss of more volatile monoterpenes from the fiber and longer exposure times (60 to 120 minutes, e.g., Calvi et al.[30]) would reduce sample throughput.
Higher extraction temperatures (145 °C) were employed for static headspace (SHS) analysis. This methodology is generally regarded as providing superior quantitation compared to SPME and has been described previously for the quantification of terpenes in cannabis.[31][32] We found that, for our purposes, it lacked sensitivity for all sesquiterpenes, despite the higher extraction temperature.
The third sample extraction and introduction trialed was liquid extraction with an organic solvent. Solvents tested were hexane, ethyl acetate, and isopropanol. While all performed equally well in terms of their extraction characteristics, isopropanol and, to a lesser extent, ethyl acetate caused peak fronting, particularly for early eluting monoterpenes (Figure 3). Hexane also afforded clear, lighter colored extracts which, in our experience, translates to lower amounts of non-target compounds and nonvolatile contaminants, resulting in higher instrument uptime and reliability. Hexane was therefore chosen for subsequent experiments. Comparing chromatograms for the three extraction strategies, it became apparent that only solvent extraction was able to provide a full representation of all relevant mono- and sesquiterpenes and -terpenoids (Figure 1). In terms of throughput, headspace techniques would be advantageous, as they require minimal sample preparation (weighing); however, the hexane extract strategy still allows for well over 100 samples to be processed within a work day, equivalent to several days’ worth of instrument time. It was therefore decided to use solvent extraction with hexane for subsequent experiments.
Next, two additional columns were evaluated for their chromatographic characteristics. The initial method development described above was carried out on a DB-5 non-polar column. Other columns evaluated from our inventory were DB-17 and VF-35, both of intermediate polarity. A 5 °C/min gradient was chosen as a trade-off between shortest possible run time and best possible resolution. The runtime achieved is shorter than that reported by Giese and colleagues using a 3.5 °C gradient[23], while also resolving five additional, later eluting sesquiterpenes, including the validation compounds guaiol and bisabolol. Furthermore, by employing column backflushing after elution of bisabolol, we can prevent cannabinoids, column bleed, and other high-boiling compounds from entering the MS, reducing contamination of the source and therefore the need for more frequent source cleaning. Using a DB-17 column may also marginally reduce the runtime, as demonstrated in Figure 2, but this would mean a trade off in terms of compound identification, as this column type is very rarely used to calculate retention times indices. Initial tests also showed it did not offer significant improvements in terms of resolution over the DB-5, which affords baseline or near-baseline separation for the validation compounds (Table 1). Notably, the resolution values here were calculated using extracted ion chromatograms (EICs) from a combination of standard and sample runs, which we believe is a more accurate representation of actual cannabis samples than presenting resolution data on standard runs alone.[24][33] The p-cymene, limonene, and eucalyptol eluted in close proximity in the total ion chromatogram (TIC), but are nonetheless resolved due to their unique quantifier ions. Furthermore, only limonene was detected in significant quantities in the available biomass. Based on the samples used for determining precision, the amount of eucalyptol and β-phellandrene was approximately 1.5% that of limonene each, while that of p-cymene was below the detection limit (cp Table 4).
A total of 49 distinct individual compounds were detected using this method. Of these, 23 were classified as monoterpenes and -terpenoids, the remainder as sesquiterpenes and -terpenoids. Compounds were assigned putative identities based on spectral library matching and retention time indexing. As far as possible, these predictions were confirmed using authentic standards. While monoterpene standards are readily available commercially, sesquiterpene standards have proven more difficult to obtain, an issue also encountered in other studies.[6][31][34] With the standards available to us, we were able to conclusively identify 18 out of the 23 monoterpenes, and 8 out of 26 sesquiterpenes, as summarized in Table 1; for a further two monoterpenes (β-phellandrene and 4thujanol) and three sesquiterpenes (bergamotene isomer 3, (−)-α selinene, and δ-guaiene), we found matching retention time indices in the NIST webbook. The α-thujene was initially identified as α-phellandrene, based on spectral library matching, but did not match the retention time of an authentic α-phellandrene standard and was assigned its current identity based on the RI published by Ascrizzi and colleagues.[28]
The method was validated for 22 compounds, as listed in Table 5, for specificity (resolution) linearity, quantitation and detection limits, accuracy (recovery), and precision. Nerolidol was removed from the validation early due to poor sensitivity. Detection and quantitation limits for the remaining 21 compounds ranged from 0.017 (LOD) and 0.052 μg/mL (LOQ) for β-pinene, to 1.144 and 3.468 μg/mL for bisabolol. The majority of compounds can be detected at less than 0.1 μg/mL in solution, equivalent to 2 ppm in biomass (w/w dry weight), and quantitated at less than 0.2 μg/mL, equivalent to 4 ppm in biomass. All LODs/LOQs were well below what is generally considered pharmacologically relevant (500 ppm)[17], and the responses were linear for the ranges tested. Using an MS/MS approach instead of acquiring scan data, as advocated by Shapira and colleagues[31], was considered initially and may provide lower detection limits; however, it may also prevent the re-examination of data for previously “unexpected” compounds.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Accuracy was determined by spiking cannabis samples with three concentrations of the validation terpenes within the linear range and adjusting for endogenous levels. Recoveries mostly fall in the acceptable 80–120% range, with the exception of isopulegol (61.8% high; 66.2% mid), βcis caryophyllene (62.0% high; 72.9% mid), and caryophyllene oxide (69% high; 76.3% mid). Interestingly, the values for the low spike concentration for these compounds do fall within the 80–120% range. The precision of the method, as determined by the %RSD for eight sample preparations, was generally between 1% and 10%, with the exception of α-terpinene (16.81% Analyst 1; 7.86% Analyst 2) and eucalyptol (20.86% Analyst 1; 13.55% Analyst 2). Four validation compounds—3-carene, p-cymene, ocimene, and nerolidol—were below detection limits entirely in the cannabis material and therefore not part of the precision determination.
Given the large concentration range of terpenes in cannabis, we consider these values acceptable for our purpose. The validation parameters obtained with this method are comparable to previously published studies that use much longer gradient times and that are therefore less suitable for high-throughput terpene profiling.[24][31] As Shapira and colleagues note[31], the actual accuracy and precision can be influenced by minor variations in sample preparation and variation in endogenous levels. Consequently, we saw the largest outlier in precision in two of the lowest level compounds.
Materials and methods
All solvents were 99% purity or better and obtained from Thermo Fisher Scientific (Scoresby, VIC, Australia). Supelco 30/50 DVB/CAR-PDMS SPME fibers were purchased from Sigma-Aldrich (Ryde, NSW, Australia).
Unless stated otherwise, individual authentic standards and standard solutions for compound identification were obtained from Sigma-Aldrich and Leco Australia (Baulkham Hills, NSW, Australia) as distributers for Restek (Bellefonte, CA, USA). Combined cannabis terpenes standard solutions #1 and #2 (CT, 2.5 mg/mL in isopropanol) were purchased from Restek. Dodecane (internal standard) and C8-C20 alkane mix were obtained from Sigma-Aldrich.
Cannabis plants were grown at an Agriculture Victoria Research indoor growth facility. Multiple cannabis strains from a variety of chemotypes were used for the study. Dried samples of inflorescence were ground to a fine powder using a SPEX SamplePrep 2010 Geno/Grinder for one minute at 1500 Hz.
In a preliminary experiment, different cannabis strains were analyzed for their volatile production and compared using principal component analysis. Chemically diverse strains were selected and combined to create a test sample that represents the largest possible variety of volatile compounds.
Headspace techniques
The 20 mg combined sample was weighed into a 20 mL headspace vial, sealed, and incubated at 40 °C for 10 minutes. For SPME analysis, a 1 cm 30/50 divinylbenzene-carboxen polydimethylsiloxane fiber (Supelco, Bellefonte, PA, USA) was incubated in the sample headspace for 30 minutes and desorbed in the GC inlet for three minutes. The fiber was reconditioned between samples.
For static headspace analysis, 1 mL of the gas phase was removed using a gas-tight headspace syringe and injected directly. Different incubation temperatures from 40–145 °C were trialed to maximize recovery of higher-boiling sesquiterpenes. The syringe was kept at 150 °C and flushed with high-purity nitrogen between samples to avoid carryover.
Liquid extracts
Of the combined sample, 40 mg was weighed into 2 mL microcentrifuge tubes, and 400 μL of extraction solvent (50 mg/L dodecane in hexane) were added. Samples were vortexed for 30 seconds, then sonicated for 10 minutes, and centrifuged at maximum g for five munutes. The supernatant was removed to an autosampler vial, the extraction procedure was repeated, and first and second extracts combined. A third extraction was used to determine extraction efficiency. Hexane, isopropanol, and ethyl acetate were evaluated as extraction solvents, and ultimately, hexane was chosen for the validation.
For measuring myrcene concentration, samples were further diluted 1:5 in hexane to bring the analyte concentration into the linear range.
Standard preparation
For compound identification, individual standards were diluted in hexane (Sigma standards) or methanol (Restek individual standards) to between 20–100 ng/μL. The combined CT standards were used for quantitation, spiking, and LOD/LOQ experiments. Dodecane and sabinene stock solutions were prepared at 10 mg/mL. CT standards #1 and #2 and sabinene stock solution were combined in hexane to obtain a solution of 100 μg/mL. This solution was then serially diluted 1:2 in hexane nine more times to obtain a lowest concentration of 0.195 μg/mL. To these, internal standard was added to a final concentration of 50 μg/mL.
GC-MS analysis
All analyses were performed on an Agilent 7890A GC with a 7000 Triple Quadrupole Mass Spectrometer and Gerstel MPS autosampler with heated agitator. The GC inlet was held at 270 °C, with a split ratio of 20:1. The injection volume was 2 μL. Column 1 was an Agilent J&W DB-5MS-DG, 30 m × 0.25 mm I.D, × 0.25 μm film thickness with an integral 10 m guard column, connected to a 2.5 m × 0.18 mm deactivated fused silica capillary (noncoated) via a purged union. Other primary columns tested were Agilent J&W DB-17 30 m × 0.25 mm, × 0.25 μm, and Varian VF-35 30 m × 0.25 mm, ×1.0 μm. Helium was the carrier gas at a constant flow of 32 cm/s for column 1 and constant pressure of 8 psi for column 2. A single linear gradient was used starting at 60 °C held for two minutes and increasing to 200 °C at 5 °C /minute. During post run time, column 1 was backflushed for five minutes (approximately five column volumes) at 320 °C.
The MS temperatures were as follows: transfer line and EI source at 280 °C; quadrupoles at 150 °C. Data were acquired in MS1 scan mode from 40–250 m/z using EI at 70eV after a five-minute solvent delay. Compounds were putatively identified by spectral matching against the NIST 2008 library and retention time indices in the NIST webbook using the method of Van den Dool and Kratz.[35] Predictions were confirmed using authentic standards where available. Quantitation was performed using Agilent MassHunter Quant software with the largest fragment ion (base peak) used for quantitation.
Validation parameters
The method was validated for resolution, linearity, quantitation and detection limits, accuracy, and precision for the compounds listed in Table 5.
Resolution (R) for the two adjacent peaks was determined using extracted ion chromatograms for the base peak ion (quantifier) of each detected compound, based on their retention times T1/T2 and peak widths w1/w2:
For determining linearity, LOD, and LOQ, a 1:2 dilution series of the combined CT and Sabinene standard, ranging from 100 μg/mL to 0.195 ng/mL, was injected. Calibration curves were required to have R2 values greater than or equal to 0.99. LOD and LOQ were determined as LOD = 3.3 × σ/S and LOQ = 10 × σ/S, with σ representing the standard deviation of the compound and S the slope of the compound’s calibration curve.
To determine method accuracy, three replicate sample preparations were spiked at 25, 12.5, and 2.5 μg/mL of CT and sabinene and extracted. Unspiked plant material was extracted to determine endogenous levels. Recoveries were determined as:
- (measured concentration − endogenous concentration)/spiked concentration × 100
Acceptable recoveries were defined as 80–120%.
Precision and intermediate precision were determined by analyzing eight individual preparations of plant material extracted independently by two analysts on different days. The relative standard deviation for the concentration of the compounds was ≤ 6% for individual analysts and ≤ 8% combined.
Conclusions
As accelerated precision breeding programs for medicinal cannabis become more widespread, accurately measuring the non-cannabinoid content in cannabis, particularly terpenes and terpenoids, has become increasingly important. Current methods, however, are unsuitable for the high-throughput analyses required for large scale breeding programs. The method presented here covers a large cross-section of commonly detected cannabis volatiles, is validated for a large proportion of compounds it covers, and offers significant improvement in terms of sample preparation and sample throughput over previously published studies.
Acknowledgements
The authors acknowledge the hard work of the AV glasshouse and cultivation team for providing medicinal cannabis biomass, Vilnis Ezernieks and Aaron Elkins for providing technical support, and Carolyn Bath for support during editing.
Author contributions
C.K., S.R. and G.S. conceptualized, supervised, and resourced the research; C.K. carried out the main experiments; C.K., S.R. and G.S. undertook the writing, editing, and review of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of interest
The authors declare no conflict of interest.
References
- ↑ Russo, E.B. (2019). "The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No "Strain," No Gain". Frontiers in Plant Science 9: 1969. doi:10.3389/fpls.2018.01969. PMC PMC6334252. PMID 30687364. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6334252.
- ↑ Russo, E.B. (2007). "History of cannabis and its preparations in saga, science, and sobriquet". Chemistry & Biodiversity 4 (8): 1614-48. doi:10.1002/cbdv.200790144. PMID 17712811.
- ↑ 3.0 3.1 3.2 Wright, R. (24 November 2017). "Cannabis Industry Report" (PDF). EverBlu Capital. https://www.everblucapital.com/wp-content/uploads/2017/11/EverBlu-Research-Cannabis-Industry-Report.pdf. Retrieved 12 January 2019.
- ↑ 4.0 4.1 Whiting, P.F.; Wolff, R.F.; Deshpande, S. et al. (2015). "Cannabinoids for Medical Use: A Systematic Review and Meta-analysis". JAMA 313 (24): 2456–73. doi:10.1001/jama.2015.6358. PMID 26103030.
- ↑ 5.0 5.1 5.2 Klumpers, L.E.; Thacker, D.L. (2019). "A Brief Background on Cannabis: From Plant to Medical Indications". Journal of AOAC International 102 (2): 412–20. doi:10.5740/jaoacint.18-0208. PMID 30139415.
- ↑ 6.0 6.1 6.2 Booth, J.K.; Bohlmann, J. (2019). "Terpenes in Cannabis sativa - From plant genome to humans". Plant Science 284: 67–72. doi:10.1016/j.plantsci.2019.03.022. PMID 31084880.
- ↑ 7.0 7.1 7.2 Pamplona, F.A.; da Silva, L.R.; Coan, A.C. (2018). "Potential Clinical Benefits of CBD-Rich Cannabis Extracts Over Purified CBD in Treatment-Resistant Epilepsy: Observational Data Meta-analysis". Frontiers in Neurology 9: 759. doi:10.3389/fneur.2018.00759. PMC PMC6143706. PMID 30258398. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6143706.
- ↑ Bonini, S.A.; Premoli, M.; Tambaro, S. et al. (2018). "Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history". Journal of Ethnopharmacology 227: 300–15. doi:10.1016/j.jep.2018.09.004. PMID 30205181.
- ↑ 9.0 9.1 Weston-Green, K. (2019). "The United Chemicals of Cannabis: Beneficial Effects of Cannabis Phytochemicals on the Brain and Cognition". In Costain, W.J.; Laprarie, R.B.. Recent Advances in Cannabinoid Research. IntechOpen. doi:10.5772/intechopen.79266. ISBN 9781838806415.
- ↑ 10.0 10.1 Baron, E.P. (2018). "Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science". Headache 58 (7): 1139–86. doi:10.1111/head.13345. PMID 30152161.
- ↑ Rohleder, C.; Müller, J.K.; Lange, B. et al. (2016). "Cannabidiol as a Potential New Type of an Antipsychotic. A Critical Review of the Evidence". Frontiers in Pharmacology 7: 422. doi:10.3389/fphar.2016.00422. PMC PMC5099166. PMID 27877130. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5099166.
- ↑ Bonn-Miller, M.O.; ElSohly, M.A.; Loflin, M.J.E. et al. (2018). "Cannabis and cannabinoid drug development: evaluating botanical versus single molecule approaches". International Review of Psychiatry 30 (3): 277–84. doi:10.1080/09540261.2018.1474730. PMC PMC6242809. PMID 30179534. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6242809.
- ↑ Ben-Shabat, S.; Fride, E.; Sheskin, T. et al. (1998). "An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity". European Journal of Pharmacology 353 (1): 23–31. doi:10.1016/s0014-2999(98)00392-6. PMID 9721036.
- ↑ Mechoulam, R.; Ben-Shabat, S. (1999). "From gan-zi-gun-nu to anandamide and 2-arachidonoylglycerol: The ongoing story of cannabis". Natural Product Reports 16 (2): 131–43. doi:10.1039/a703973e. PMID 10331283.
- ↑ 15.0 15.1 McPartland, J.M.; Russo, E.B. (2001). "Cannabis and Cannabis Extracts: Greater Than the Sum of Their Parts?". Journal of Cannabis Therapeutics 1 (3–4): 103–32. doi:10.1300/J175v01n03_08.
- ↑ 16.0 16.1 Santiago, M.; Sachdev, S.; Arnold, J.C. etc. (2019). "Absence of Entourage: Terpenoids Commonly Found in Cannabis sativa Do Not Modulate the Functional Activity of Δ 9-THC at Human CB 1 and CB 2 Receptors". Cannabis and Cannabinoid Research 4 (3): 165-176. doi:10.1089/can.2019.0016. PMC PMC6757242. PMID 31559333. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6757242.
- ↑ 17.0 17.1 17.2 Russo, E.B. (2011). "Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects". British Journal of Pharmacology 163 (7): 1344–64. doi:10.1111/j.1476-5381.2011.01238.x. PMC PMC3165946. PMID 21749363. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3165946.
- ↑ Kamal, B.S.; Kamal, F.; Lantela, D.E. (2018). "Cannabis and the Anxiety of Fragmentation-A Systems Approach for Finding an Anxiolytic Cannabis Chemotype". Frontiers in Neuroscience 12: 730. doi:10.3389/fnins.2018.00730. PMC PMC6204402. PMID 30405331. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6204402.
- ↑ Adams, T.B.; Taylor, S.V. (2010). "Safety evaluation of essential oils: A constituent-based approach". In Baser, K.H.C.; Buchbauer, G.. Handbook of Essential Oils: Science, Technology, and Applications. CRC Press. pp. 185–208. ISBN 9781420063158.
- ↑ 20.0 20.1 Nuutinen, T. (2018). "Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus". European Journal of Medicinal Chemistry 157: 198–228. doi:10.1016/j.ejmech.2018.07.076. PMID 30096653.
- ↑ do Vale, T.G.; Furtado, E.C.; Santos Jr., J.G. et al. (2002). "Central effects of citral, myrcene and limonene, constituents of essential oil chemotypes from Lippia alba (Mill.) n.e. Brown". Phytomedicine 9 (8): 709–14. doi:10.1078/094471102321621304. PMID 12587690.
- ↑ 22.0 22.1 Leghissa, A.; Hildenbrand, Z.L.; Schug, K.A. (2018). "A review of methods for the chemical characterization of cannabis natural products". Journal of Separation Science 41 (1): 398-415. doi:10.1002/jssc.201701003. PMID 28986974.
- ↑ 23.0 23.1 Giese, M.W.; Lewis, M.A.; Giese, L. et al. (2015). "Development and Validation of a Reliable and Robust Method for the Analysis of Cannabinoids and Terpenes in Cannabis". Journal of AOAC International 98 (6): 1503–22. doi:10.5740/jaoacint.15-116. PMID 26651562.
- ↑ 24.0 24.1 24.2 Ibrahim, E.A.; Wang, M.; Radwan, M.M. et al. (2019). "Analysis of Terpenes in Cannabis sativa L. Using GC/MS: Method Development, Validation, and Application". Planta Medica 85 (5): 431–38. doi:10.1055/a-0828-8387. PMID 30646402.
- ↑ Fischedick, J.T.; Hazekamp, A.; Ekelens, T. et al. (2010). "Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes". Phytochemistry 71 (17–18): 2058–73. doi:10.1016/j.phytochem.2010.10.001. PMID 21040939.
- ↑ Fischedick, J.T. (2017). "Identification of Terpenoid Chemotypes Among High (-)- trans-Δ 9- Tetrahydrocannabinol-Producing Cannabis sativa L. Cultivars". Cannabis and Cannabinoid Research 2 (1): 34–47. doi:10.1089/can.2016.0040. PMC PMC5436332. PMID 28861503. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5436332.
- ↑ Kolb, B.; Ettre, L.S. (2006). Static Headspace-Gas Chromatography: Theory and Practice (2nd ed.). John Wiley & Sons. ISBN 978047174944-8.
- ↑ 28.0 28.1 Ascrizzi, R.; Flamini, G.; Guisiani, M. et al. (2018). "VOCs as fingerprints for the chemical profiling of hashish samples analyzed by HS-SPME/GC–MS and multivariate statistical tools". Forensic Toxicology 36: 243–60. doi:10.1007/s11419-017-0398-1.
- ↑ Rice, S.; Koziel, J.A. (2015). "Characterizing the Smell of Marijuana by Odor Impact of Volatile Compounds: An Application of Simultaneous Chemical and Sensory Analysis". PLoS One 10 (12): e0144160. doi:10.1371/journal.pone.0144160. PMC PMC4684335. PMID 26657499. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4684335.
- ↑ Calvi, L.; Pentimalli, D.; Panseri, S. et al. (2018). "Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC-MS and LC-HRMS (q-exactive orbitrap®) approach". Journal of Pharmaceutical and Biomedical Analysis 150: 208–19. doi:10.1016/j.jpba.2017.11.073. PMID 29247961.
- ↑ 31.0 31.1 31.2 31.3 31.4 Shapira, A.; Berman, P.; Futoran, K. et al. (2019). "Tandem Mass Spectrometric Quantification of 93 Terpenoids in Cannabis Using Static Headspace Injections". Analytical Chemistry 91 (17): 11425–32. doi:10.1021/acs.analchem.9b02844. PMID 31369251.
- ↑ Snow, N.H.; Bullock, G.P. (2010). "Novel techniques for enhancing sensitivity in static headspace extraction-gas chromatography". Journal of Chromatography A 1217 (16): 2726–35. doi:10.1016/j.chroma.2010.01.005. PMID 20116067.
- ↑ "Method development for qualification and quantification of cannabinoids and terpenes in extracts by gas chromatography-mass spectrometry". Master's Thesis. University of Texas Arlington. 19 July 2016. http://hdl.handle.net/10106/26115.
- ↑ Leghissa, A.; Hildenbrand, Z.L.; Foss, F.W. et al. (2018). "Determination of cannabinoids from a surrogate hops matrix using multiple reaction monitoring gas chromatography with triple quadrupole mass spectrometry". Journal of Separation Science 41 (2): 459-468. doi:10.1002/jssc.201700946. PMID 29094798.
- ↑ Van den Dool, H.; Kratz, P.D. (1963). "A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography". Journal of Chromatography 11: 436–71. doi:10.1016/s0021-9673(01)80947-x. PMID 14062605.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. The original includes an "in prep" citation at citation 8, but the citation appears to be about grapes and tobacco and not related to cannabis; it could not be verified as published and has been omitted for this version.