Difference between revisions of "Journal:Cannabis contaminants limit pharmacological use of cannabidiol"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (Finished adding rest of content.) |
||
| Line 19: | Line 19: | ||
|download = [https://www.frontiersin.org/articles/10.3389/fphar.2020.571832/pdf https://www.frontiersin.org/articles/10.3389/fphar.2020.571832/pdf] (PDF) | |download = [https://www.frontiersin.org/articles/10.3389/fphar.2020.571832/pdf https://www.frontiersin.org/articles/10.3389/fphar.2020.571832/pdf] (PDF) | ||
}} | }} | ||
==Abstract== | ==Abstract== | ||
For nearly a century, [[wikipedia:Cannabis|cannabis]] has been stigmatized and [[wikipedia:Legality of cannabis|criminalized]] across the globe, but in recent years, there has been a growing interest in cannabis due to the therapeutic potential of [[wikipedia:Cannabinoid#Phytocannabinoids|phytocannabinoids]]. With this emerging interest in cannabis, concerns have arisen about the possible [[wikipedia:Contamination|contaminations]] of [[wikipedia:Hemp|hemp]] with [[wikipedia:Pesticide|pesticides]], [[wikipedia:Heavy metals|heavy metals]], microbial [[wikipedia:Pathogen|pathogens]], and [[wikipedia:Carcinogen|carcinogenic]] compounds during the [[wikipedia:Cannabis cultivation|cultivation]], manufacturing, and packaging processes. This is of particular concern for those turning to cannabis for [[wikipedia:Cannabis (drug)|medicinal purposes]], especially those with compromised immune systems. This review aims to provide types of contaminants and examples of cannabis contamination using case studies that elucidate the medical consequences consumers risk when using adulterated cannabis products. Thus, it is imperative to develop universal standards for cultivation and [[LII:Past, Present, and Future of Cannabis Laboratory Testing and Regulation in the United States|testing]] of products to protect those who consume cannabis. | For nearly a century, [[wikipedia:Cannabis|cannabis]] has been stigmatized and [[wikipedia:Legality of cannabis|criminalized]] across the globe, but in recent years, there has been a growing interest in cannabis due to the therapeutic potential of [[wikipedia:Cannabinoid#Phytocannabinoids|phytocannabinoids]]. With this emerging interest in cannabis, concerns have arisen about the possible [[wikipedia:Contamination|contaminations]] of [[wikipedia:Hemp|hemp]] with [[wikipedia:Pesticide|pesticides]], [[wikipedia:Heavy metals|heavy metals]], microbial [[wikipedia:Pathogen|pathogens]], and [[wikipedia:Carcinogen|carcinogenic]] compounds during the [[wikipedia:Cannabis cultivation|cultivation]], manufacturing, and packaging processes. This is of particular concern for those turning to cannabis for [[wikipedia:Cannabis (drug)|medicinal purposes]], especially those with compromised immune systems. This review aims to provide types of contaminants and examples of cannabis contamination using case studies that elucidate the medical consequences consumers risk when using adulterated cannabis products. Thus, it is imperative to develop universal standards for cultivation and [[LII:Past, Present, and Future of Cannabis Laboratory Testing and Regulation in the United States|testing]] of products to protect those who consume cannabis. | ||
| Line 114: | Line 109: | ||
[[wikipedia:Polycyclic aromatic hydrocarbon|Polycyclic aromatic hydrocarbons]] (PAHs) are ubiquitous environmental pollutants usually generated by the incomplete combustion of organic materials (e.g., oil, coal, and wood).<ref name="Abdel-ShafyARev16">{{cite journal |title=A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation |journal=Egyptian Journal of Petroleum |author=Abdel-Shafy, H.I.; Mansour, M.S.M. |volume=25 |issue=1 |pages=107–23 |year=2016 |doi=10.1016/j.ejpe.2015.03.011}}</ref> They are found in some CBD oils and may come either from uptake by the plant during growth or from contaminated carrier oils during product preparation.<ref name="VečerkaWarn18">{{cite web |url=https://www.icci.science/en/article/news/warning-for-consumers-of-cbd-and-cannabis-oils-sold-on-the-eu-market/ |title=Warning for consumers of CBD and cannabis oils sold on the EU market |author=Večerka, J. |publisher=International Cannabis and Cannabinoids Institute |date=16 February 2018 |accessdate=22 January 2020}}</ref> Excessive PAH content in CBD oils can be attributed to the smoke from nearby forest fires or from drying cannabis with propane heaters.<ref name="ZelinkovaTheOcc15">{{cite journal |title=The Occurrence of 16 EPA PAHs in Food - A Review |journal=Polycyclic Aromatic Compounds |author=Zelinkova, Z.; Wenzl, T. |volume=35 |issue=2–4 |pages=248–84 |year=2015 |doi=10.1080/10406638.2014.918550 |pmid=26681897 |pmc=PMC4673601}}</ref> Over 100 types of PAHs exist, and some of the most studied and well characterized (i.e., benzo anthracene, chrysene, benzo fluoranthene, benzo pyrene) are known to be hazardous carcinogens, and can found in cannabis products worldwide. In the E.U., 20 out of 29 tested CBD oil brands were shown to have PAH levels higher than the legislative limits of 20 mg/kg.<ref name="WhiteARev19">{{cite journal |title=A Review of Human Studies Assessing Cannabidiol's (CBD) Therapeutic Actions and Potential |journal=Journal of Clinical Pharmacology |author=White, C.M. |volume=59 |issue=7 |pages=923–34 |year=2019 |doi=10.1002/jcph.1387 |pmid=30730563}}</ref> High levels of PAHs in CBD oil are likely to cause DNA methylation, DNA adducts, and alteration of histone methylation, which can lead to immunosuppression.<ref name="Abdel-ShafyARev16" /> The elimination of PAHs in the environment is most studied in biological systems through multi-step metabolic pathways, primarily mixed-function oxidase systems, but they can degrade through oxidation reactions in the environment.<ref name="Abdel-ShafyARev16" /> The extent to how any given PAH is eliminated is highly dependent on its unique physical and chemical properties.<ref name="Abdel-ShafyARev16" /> While it may be impossible to eliminate PAHs in cannabis products due to the ubiquitous nature of PAHs in the environment and the risk of producing these compounds when smoking cannabis, consumer exposure can be reduced by addressing the sources of contamination and avoiding growing cannabis in heavily industrialized areas.<ref name="JettCanna18">{{cite journal |title=Cannabis Use, Lung Cancer, and Related Issues |journal=Journal of Thoracic Oncology |author=Jett, J.; Stone, E.; Warren, G. et al. |volume=13 |issue=4 |pages=480–487 |year=2018 |doi=10.1016/j.jtho.2017.12.013 |pmid=29374567}}</ref> | [[wikipedia:Polycyclic aromatic hydrocarbon|Polycyclic aromatic hydrocarbons]] (PAHs) are ubiquitous environmental pollutants usually generated by the incomplete combustion of organic materials (e.g., oil, coal, and wood).<ref name="Abdel-ShafyARev16">{{cite journal |title=A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation |journal=Egyptian Journal of Petroleum |author=Abdel-Shafy, H.I.; Mansour, M.S.M. |volume=25 |issue=1 |pages=107–23 |year=2016 |doi=10.1016/j.ejpe.2015.03.011}}</ref> They are found in some CBD oils and may come either from uptake by the plant during growth or from contaminated carrier oils during product preparation.<ref name="VečerkaWarn18">{{cite web |url=https://www.icci.science/en/article/news/warning-for-consumers-of-cbd-and-cannabis-oils-sold-on-the-eu-market/ |title=Warning for consumers of CBD and cannabis oils sold on the EU market |author=Večerka, J. |publisher=International Cannabis and Cannabinoids Institute |date=16 February 2018 |accessdate=22 January 2020}}</ref> Excessive PAH content in CBD oils can be attributed to the smoke from nearby forest fires or from drying cannabis with propane heaters.<ref name="ZelinkovaTheOcc15">{{cite journal |title=The Occurrence of 16 EPA PAHs in Food - A Review |journal=Polycyclic Aromatic Compounds |author=Zelinkova, Z.; Wenzl, T. |volume=35 |issue=2–4 |pages=248–84 |year=2015 |doi=10.1080/10406638.2014.918550 |pmid=26681897 |pmc=PMC4673601}}</ref> Over 100 types of PAHs exist, and some of the most studied and well characterized (i.e., benzo anthracene, chrysene, benzo fluoranthene, benzo pyrene) are known to be hazardous carcinogens, and can found in cannabis products worldwide. In the E.U., 20 out of 29 tested CBD oil brands were shown to have PAH levels higher than the legislative limits of 20 mg/kg.<ref name="WhiteARev19">{{cite journal |title=A Review of Human Studies Assessing Cannabidiol's (CBD) Therapeutic Actions and Potential |journal=Journal of Clinical Pharmacology |author=White, C.M. |volume=59 |issue=7 |pages=923–34 |year=2019 |doi=10.1002/jcph.1387 |pmid=30730563}}</ref> High levels of PAHs in CBD oil are likely to cause DNA methylation, DNA adducts, and alteration of histone methylation, which can lead to immunosuppression.<ref name="Abdel-ShafyARev16" /> The elimination of PAHs in the environment is most studied in biological systems through multi-step metabolic pathways, primarily mixed-function oxidase systems, but they can degrade through oxidation reactions in the environment.<ref name="Abdel-ShafyARev16" /> The extent to how any given PAH is eliminated is highly dependent on its unique physical and chemical properties.<ref name="Abdel-ShafyARev16" /> While it may be impossible to eliminate PAHs in cannabis products due to the ubiquitous nature of PAHs in the environment and the risk of producing these compounds when smoking cannabis, consumer exposure can be reduced by addressing the sources of contamination and avoiding growing cannabis in heavily industrialized areas.<ref name="JettCanna18">{{cite journal |title=Cannabis Use, Lung Cancer, and Related Issues |journal=Journal of Thoracic Oncology |author=Jett, J.; Stone, E.; Warren, G. et al. |volume=13 |issue=4 |pages=480–487 |year=2018 |doi=10.1016/j.jtho.2017.12.013 |pmid=29374567}}</ref> | ||
==Other foreign matter== | |||
Other debris such as metal fragments, hairs, dusts, machine oils, or insect parts can be found in some CBD oil products, as can be seen in other foods or food products.<ref name="DryburghCanna18" /> The FDA considers these foreign contaminants a negligible health hazard, but clearly this needs to be addressed by manufactures to develop high-quality control standards required to limit and minimize any foreign matter contamination. | |||
==Discussion== | |||
With the recent legalization of cannabis in many states of the U.S., there have been several state regulatory commissions put into place that address the issue of quality control in terms of contaminants and the cannabinoid profile. However, cannabis testing requirements do vary from state to state in terms of the minimum number of contaminants that must be tested for, and only 15 states currently have a regulatory commission in place. For the safety and welfare of all users, both medicinal and recreational, there is a necessity for a standardized set of guidelines for cultivation and testing of cannabis products. There is currently only one set of guidelines—developed by the American Herbal Products Association—called ''Recommendations for Regulators — Cannabis Operations'' that provides a detailed set of recommended instructions on cultivation, packaging, testing, and dispensing of cannabis products (both THC and CBD products), which has proven invaluable for ensuring the safe cultivation of cannabis.<ref name="AHPARecomm16">{{cite web |url=http://www.ahpa.org/Portals/0/PDFs/Committee/CC/Cannabis_Operations_Recommendations_Regulators.pdf |format=PDF |title=Recommendations for Regulators - Cannabis Operations |author=American Herbal Products Association |publisher=American Herbal Products Association |date=02 February 2020}}</ref> While these are a great set of guidelines, a more comprehensive understanding of the contamination of cannabis products is necessary to appropriately eliminate the possible deleterious health effects contaminates may cause. Unfortunately, the classification of cannabis as a [[wikipedia:Controlled Substances Act#Schedules of controlled substances|Schedule I]] drug federally makes the development and implementation of nationwide standards impossible at the moment, which, if left unchanged, could lead to significant health complications in those turning to cannabis for its medicinal properties. | |||
==Acknowledgements== | |||
===Author contributions=== | |||
S-HP and BVH conceived the review idea. MC and ZM wrote the initial draft. S-HP and CP reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version. | |||
===Funding=== | |||
This study was funded by the Institute of Cannabis Research (ICR). | |||
===Conflict of interest=== | |||
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. | |||
==References== | ==References== | ||
Latest revision as of 19:14, 15 September 2020
| Full article title | Cannabis contaminants limit pharmacological use of cannabidiol |
|---|---|
| Journal | Frontiers in Pharmacology |
| Author(s) | Montoya, Zackary; Conroy, Matthieu; Vanden Heuvel, Brian D.; Pauli, Christopher S.; Park, Sang-Hyuck |
| Author affiliation(s) | Colorado State University–Pueblo |
| Primary contact | Email: sanghyuck dot park at csupueblo dot edu |
| Editors | Khan, Tanveer A. |
| Year published | 2020 |
| Volume and issue | 11 |
| Article # | 571832 |
| DOI | 10.3389/fphar.2020.571832 |
| ISSN | 1663-9812 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.frontiersin.org/articles/10.3389/fphar.2020.571832/full |
| Download | https://www.frontiersin.org/articles/10.3389/fphar.2020.571832/pdf (PDF) |
Abstract
For nearly a century, cannabis has been stigmatized and criminalized across the globe, but in recent years, there has been a growing interest in cannabis due to the therapeutic potential of phytocannabinoids. With this emerging interest in cannabis, concerns have arisen about the possible contaminations of hemp with pesticides, heavy metals, microbial pathogens, and carcinogenic compounds during the cultivation, manufacturing, and packaging processes. This is of particular concern for those turning to cannabis for medicinal purposes, especially those with compromised immune systems. This review aims to provide types of contaminants and examples of cannabis contamination using case studies that elucidate the medical consequences consumers risk when using adulterated cannabis products. Thus, it is imperative to develop universal standards for cultivation and testing of products to protect those who consume cannabis.
Keywords: cannabis, cannabidiol, cannabis contaminants, hemp, phytocannabinoids
Introduction
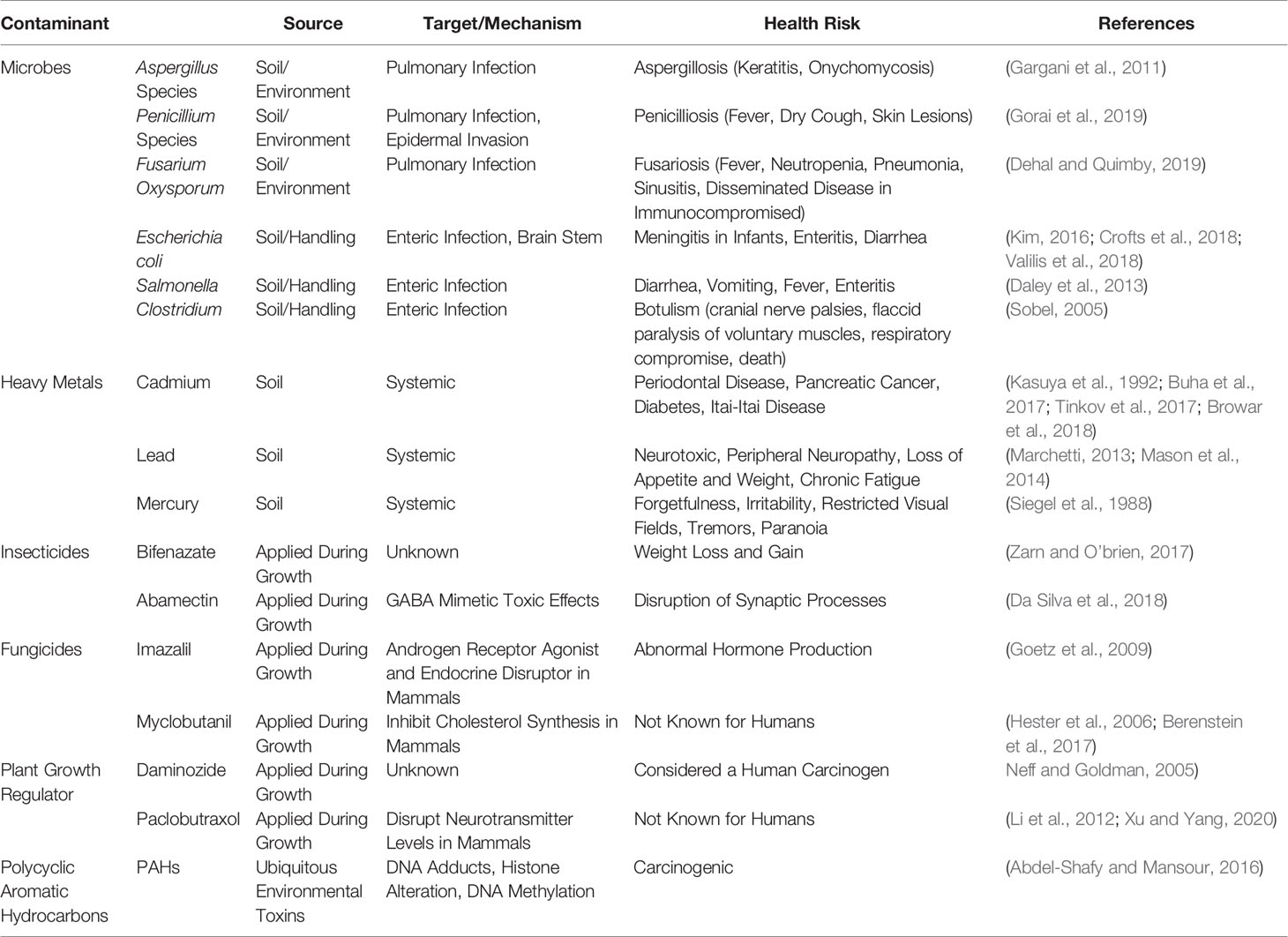
Phytocannabinoids have garnered global attention recently due to the therapeutic potentials in Parkinson’s disease[1], schizophrenia[2], cancers[3][4], pain, anxiety, depression, and other neurological disorders[5], as well as the Food and Drug Administration (FDA) approval of Epidiolex for Dravet syndrome[6] and Lennox-Gauss Syndrome.[7] As of 2019, a total of 33 states, the District of Columbia, Guam, Puerto Rico, and the U.S Virgin Islands have approved cannabis for medicinal purposes, and 21 states are considering bills that would decriminalize it under legislative action. With recent legalization in Canada in 2019, more countries are beginning to question the rationale behind criminalizing cannabis.[8] As interest in cannabis expands around the globe, many issues have arisen concerning the lack of cultivation standards and overall quality control of cannabis products. Recently the United States Pharmacopeia (USP) formed a Cannabis Expert Panel, which has evaluated specifications necessary to define key cannabis quality attributes, including limits for contaminants such as pesticide residues, microbial pathogen levels, mycotoxins, and elemental contaminants, based on toxicological considerations and aligned with the existing USP procedures for general tests and assays.[9] Aside from inaccuracy in labeling phytocannabinoid content, it has been reported that cannabis and derived products are often contaminated by microbes, heavy metals, pesticides, carcinogens, and debris, which must be addressed to ensure the safety of consumers (Table 1).[10][11]
|
These contaminants are imminent threats that directly impact public health and wellness, particularly to the immunocompromised and pediatric patients who take cannabis products as a treatment for numerous human disorders, including cancer patients and those suffering from epileptic seizures.[12] To increase public awareness, we provide examples of contamination, its medical consequences reported in clinical research, and then suggest that each risk category be analyzed for best practices to limit exposure of contaminants to the consumer. We recommend hemp producers, manufacturers, medical professionals, and legislators recognize this risk and establish regulatory measures to educate the public and lessen the adverse effects caused by the contaminants in cannabis, particularly in cannabidiol (CBD)-based products.
Labeling inaccuracy
Mislabeling of phytocannabinoid profiles in CBD products is one of the major concerns to consumers.[13] Inaccurate reporting of the cannabinoid content risks exposing medicinal users to phytocannabinoids of which they have no intent to consume, namely Δ-9-tetrahydracannabinol (THC).[14] This is of particular concern within pediatric patients, as THC intoxication has been shown to alter development of white matter in the brain[15], affect cognitive functioning[16][17], and affect learning and memory within adolescents.[18]
Despite some U.S. states like Colorado having a 15% allowable reporting variance of the phytocannabinoid content on CBD product labels[19], measured contents often exceed this range. For example, a recent study shows that 69% of 84 CBD products purchased from 31 American online retailers were inaccurately labeled for CBD: 26% were overlabeled, whereas 42% were underlabeled for CBD concentration.[20] Additionally, 64% of 14 CBD products sold in the European Union (EU) market presented different cannabinoid profiles from the declared amount.[21] Inaccuracies are also found on labels of hemp-type cannabis sold in the Netherlands, with measured THC and CBD deviating from label claims by 8%–99% in CBD oil samples obtained from patients.[13] In Germany, an analysis of 67 CBD product samples found that 25% of samples were contaminated with residual THC above the lowest level of observable effects, or the lowest level that is known to cause physiological effects in humans (2.5 mg/day).[22] In a recent analysis of 25 CBD oil products purchased in Mississippi, only three of the 25 were within ±20% of label claim, 15 were below the stated claim for CBD, two exceed these claims by more than 50%, and THC content for three products exceeded the 0.3% legal limit.[23]
There are also concerns for edible cannabis products (e.g., gummies, cookies, etc.) containing under- and overreported phytocannabinoid content, specifically THC.[24] In states where cannabis is legal for recreation, these edible products are tested for overall THC potency in addition to dose-specific potency of THC to be sure these products stay under 100 mg total THC with no more than 10 mg of THC per dose.[25] However, in hemp, there is no regulating body overseeing this testing, therefore the responsibility to test CBD edibles is left to each product manufacturer to ensure compliance of cannabinoid content limits, which is often neglected.[25] While currently CBD and THC are the only cannabinoids required to be labeled, it may be beneficial to include the profile of acidic forms of THC and CBD, as well as some representative minor cannabinoids such as cannabigerol (CBG), cannabichromene (CBC), or possibly some of the short chain versions of these referred to as "-varin cannabinoids" (e.g., tetrahydrocannabivarin [THCV] and cannabidivarin [CBDV]) on these labels. These minor cannabinoids are shown to have some therapeutic effects that could be enhanced in combination with other major cannabinoids.[26]
Microbial contamination
Cannabis is associated with various types of microbes, including molds that have been shown to harm immunocompromised patients, as well as bacteria and viruses that have the potential of causing harm to humans. A recent metagenomics study on 15 medicinal Cannabis plants shows that cannabis is associated with a wide range of epiphytic and endophytic microbial communities, including several toxigenic bacterial and fungal species.[27] While most of the microbes found to be in association with cannabis are likely beneficial to the plant in some way or are phytopathogens, several bacterial species have been identified that could be opportunistic pathogens in humans.[27] While there are currently no reports of bacterial infection caused by contaminated cannabis, several examples of fungal contamination, namely the Aspergillus species, are found in the literature and pose a threat to human health.[27] In this section, the authors will introduce some of the possible human pathogenic microbial species and their relevant case studies.
Fungal contaminants
Previous studies have identified several fungal organisms in dispensary-produced cannabis, including species of Penicillium (P. paxilli, P. citrinum, P. commune, P. chrysogenum, P. corylophilum, P. citrinum, and P. steckii), Aspergillus (A. terreus, A. niger, A. flavus, A. versicolor, A. ostianus, and A. sydowii), and Fusarium (F. oxysporum).[27][28] Both Penicillium and Aspergillus species have been known to produce aflatoxins (e.g., aflatoxin B1), while Fusarium species produce other mycotoxins such as fumonisin.[29][30] Cannabis infected with Aspergillus, Penicilium, or Fusarium can severely affect human health as these toxins can all be carcinogenic, hepatotoxic, neurotoxic or nephrotoxic.[31][32][33] The toxicological action depends on various factors, including the mode of exposure and the susceptibility of the infected individual, with immunocompromised patients having the highest risk of infection.[31] In general, these toxins are carcinogenic as they commonly interact with guanine moieties in DNA forming a variety of DNA adducts, which often leads to deterioration of the liver.[31]
Case study 1
Penicilliosis (today known as talaromycosis), a fungal infection due to Penicillium species, is rare in immunocompetent people but is found in immunocompromised individuals[34], and it is commonly the cause of death for human immunodeficiency virus (HIV) positive and other immunocompromised patients.[35] Currently, no reports of penicilliosis caused by cannabis are found in the literature; however, immunocompromised individuals should be cautious using cannabis products as several species known to cause this condition have been found in cannabis flowers and products.[27][28]
Case study 2
Aspergillus species are the most common fungi to cause invasive infection in the immunocompromised. This is concerning as Aspergillus-infected cannabis has been previously directly linked to human disease.[36] A case study showed that a patient with lung cancer used illicitly obtained cannabis as an antiemetic agent during chemotherapy and developed invasive pulmonary aspergillosis that caused death in 19 days after diagnosis.[37] Many of the recent metagenomic studies of cannabis show that Aspergillus species are still pervasive in cannabis, which may pose a considerable risk to the consumer, especially the immunocompromised.[27]
Case study 3
Fusarium species are common environmental fungi, capable of causing infections in both animals and plants.[33] Humans infected by Fusarium present with a wide range of symptoms, including fever, neutropenia, pneumonia, sinusitis, or, in some immunocompromised patients, disseminated disease.[38] Fusarium can cause a pulmonary infection that could result from the inhalation of conidia[39], a spore produced by these asexual fungi, which is consistent with the following case. A 2019 case study in an immunocompromised patient with acute myeloid leukemia (AML), who developed invasive disseminated fusariosis, has proven infection by this fungus is fatal.[38] The patient initially presented with painless lesions on her arms and legs, that darkened, grew, and spread to her trunk and all extremities. The patient elected to discontinue treatment and passed two weeks after transitioning to hospice care.[38]
There are limited case studies demonstrating cannabis causing fusariosis; however, there are a plethora of studies that have found Fusarium to be in direct relationship with Cannabis plants.[28][30] In fact, starting in the late 1970s through the 1980s, F. oxysporum was physically distributed across the United States to combat illegal cannabis farming.[28] While this was intended as a short-term biological control, it has inevitably caused this organism to continually infect legal hemp and cannabis farms today, which may negatively impact the quality of cannabis grown in legal markets.
In addition to pathogenesis in humans by these fungi, Penicillium, Aspergillus, and Fusarium species are known to produce both aflatoxins and mycotoxins that become especially problematic while drying and storing cannabis products in humid environments.[10][40] Several cannabis drying strategies, such as sweat curing, make samples more susceptible to contamination from various types of Aspergillus because of relatively high water activity inside the stacked plant materials.[10] Sweat curing is not as commonly practiced today; however, there have still been recent reports of unacceptable levels of fungal spores in products grown in both indoor and outdoor facilities.[10] This indicates some current methods of cultivation and curing still leave the plant susceptible to fungal infection.[41] As such, standard testing procedures of fungal mycotoxins in cannabis for both the hemp- and drug-type markets must be developed and are imperative to best protect the consumer, especially those with a compromised immune system using cannabis as a therapy.
Bacterial contaminants
Bacterial contamination is less of a direct health threat to cannabis users than fungus and molds, but there have been potentially pathogenic species identified in a few recent studies.[10][27][42] A study of five cannabis cultivars had shown that most species of bacteria were identified from samples of endorhiza-, rhizosphere-, and bulk soil-associated microbiomes more so than from other regions of the plant. These bacteria contaminates include various species of Pseudomonas, Cellvibrio, Oxalobacteraceae, Xanthomonadaceae, Actinomycetales, and Sphingobacteriales in the examined microbiomes.[43] Another study shows a variety of potential human pathogens, including Acinetobacter baumannii, Escherichia coli, Pseudomonas aeruginosa, Ralstonia pickettii, Salmonella enterica, Stenotrophomonas maltophilia, and Clostridium botulinum, in the flowers of medicinal cannabis plants grown at indoor facilities in Massachusetts, Maine, and Rhode Island.[27] Endophytic bacterial taxa have also been identified that may provide fungal resistance and other fitness-related traits to cannabis through secondary metabolite production, some of which could be used in growth promotion and/or in biological control designed experiments.[44] Although some bacteria have been shown to be beneficial to cultivation, the possible pathogenic species that have been associated with cannabis are of greater concern, specifically the risk these species pose to consumers.
While dozens of bacterial species have been found to be present in cannabis plants, E. coli, Salmonella, and Clostridium are a few common potential human pathogenic species shown to be associated with cannabis.[27] Escherichia coli infection has potential to cause a wide range of diseases depending on the strain encountered, including meningitis in infants, enteritis, and diarrhea.[45][46][47] Exposure to Salmonella can cause bacterial infection with symptoms including diarrhea, vomiting, fever, and enteritis.[29] Clostridium can cause botulism, a rare disease with symptoms including cranial nerve palsies and flaccid paralysis of voluntary muscles, with potential progression to respiratory illness and death.[48]
There are also concerns for the contamination of cannabis food products by potentially harmful bacteria, including Listeria.[49] Species of Listeria have been shown to be opportunistic pathogens that most commonly cause food poisoning or listeriosis; however, if infecting the central nervous system, these bacterium can induce encephalitis or mimic idiopathic inflammatory demyelinating disease.[50] Though the presence of these bacteria have been reported as highly prevalent in cannabis[10], current literature does not reflect any opportunistic infection caused by use of bacterial-contaminated cannabis products. Still, the presence of human pathogenic bacteria on cannabis presents a possible risk to the consumer, especially the immunocompromised, and therefore ways to limit bacterial contamination should be explored.
Viral contaminants
Our literature searches yielded no reports of human pathogenic viral contamination of cannabis, but other crops have shown contamination by various noroviruses, rotaviruses, and enteroviruses, causing enteric diseases in humans.[51][52] Viruses found to be associated with cannabis are purely plant pathogens, and it is not assumed that these could cause human related diseases.[10] However, human handling in this industry is frequent, and it is possible that the product could be contaminated with a human pathogen through contact. While no reported cases of viral infection caused by cannabis use are found in the literature, this is not a largely explored area of research and should be considered in future studies. It is possible that human viral pathogens will be identified through further metagenomic studies of cannabis, and until the risk of disease can be ruled out, viral contamination should be considered possible.
Heavy metal contamination
A variety of heavy metals have been found in Cannabis plants and products made with cannabis (e.g., tinctures and oils), including cadmium, lead, mercury, magnesium, and copper.[10][11][53][54][55] Cannabis plants have been shown to hyperaccumulate and incorporate these metals into tissues throughout the plant and have been previously explored for their ability to bioremediate contaminated soils.[10] Most heavy metals have low biodegradability, which allows them to bioaccumulate up the food chain and persist in the body long-term, causing a wide range of health problems.[56] Furthermore, many heavy metals have been shown to have fatal effects in humans when exposed both acutely or chronically, causing a plethora of diseases, such as cancers and neurological disorders.[29] While Colorado and California do require heavy metal testing in cannabis, without similar requirements in place to test for such heavy metal contamination in hemp and CBD products, many people are at risk of exposure to toxic levels of heavy metals. At the greatest risk for detrimental effects from heavy metal contamination are those using CBD as a medical treatment, including children suffering from pediatric epilepsy and the various conditions leading to compromised immune systems. Thus, the authors aim to identify medical consequences of exposure to three major heavy metal contaminants found in cannabis (i.e., cadmium, lead, and mercury) with a corresponding case study for each.
Cadmium
Cadmium is a soft, bluish-white heavy metal that is mainly obtained from zinc ore processing. It has a long biological half-life of 14 to 24 years, and bioaccumulates in the human body when chronically exposed.[57] The impact of exposure to cadmium-containing products and cadmium-containing fertilizers on humans remains a major concern.[58] Leafy and root vegetables, grains, and tobacco bioconcentrate cadmium from the soil, resulting in exposure through diet and smoking.[58] Cadmium is generally found in higher levels in urine, blood, fat, and lung tissues of tobacco smokers, which correlates with length of time as a smoker.[57] It has also been shown that application of phosphate fertilizers targeted for cannabis growth increases the uptake of cadmium by cannabis when grown in cadmium-contaminated soils, though the mechanism is not yet clear.[59] Currently, no cases of cadmium-contaminated cannabis causing health problems are found in the literature; however, there are several diseases that have been associated with exposure to cadmium through smoking and diet, including periodontal disease[60], pancreatic cancer[61], and diabetes.[62] Most severely, chronic exposure causes Itai-Itai disease, which is characterized by intense bone pain, a disrupted gait, and numbness in all extremities.[63] Cannabis products are not likely to be contaminated enough to cause disease as severe as Itai-Itai disease, but because cannabis can hyperaccumulate cadmium, it should still be considered hazardous and tested for in cannabis products.
Lead
Lead is a silver to dark gray, soft, malleable, corrosion-resistant heavy metal and is one of the earliest metals discovered.[64] Lead has been used in automobile, paint, ceramic, and plastic manufacturing, and because lead is nonbiodegradable, it persists in the environment.[64] Lead can have a variable biological half-life from 30 days for lead in the blood and up to 30 years for lead deposited into bone, which is usually a sign of chronic exposure.[65] Not only has lead been found in cannabis, but also the uptake of lead has been shown to increase in cannabis grown in contaminated soils[59], especially in contaminated urban environments.[66] Once lead enters the body, it can interact with almost every organ; however, its effects on the central nervous system are the most severe.[65] Lead acts as a calcium analog interfering with ion channels of mammalian neurons.[5] It has also been observed that lead is a potent reversible and selective blocker of voltage-dependent calcium channels even at low concentrations in human neurons.[5][65] Lead contamination of cannabis products sold in legal markets will likely be due to the cultivation of cannabis in contaminated soils, but lead has been deliberately added to cannabis as well. This is highlighted by an incident of massive lead poisoning in Leipzig, Germany where lead was intentionally added to cannabis attempting to increase its mass and in turn its street value, which caused 35 people to be treated for blood lead levels up to 1,063 µg/L.[54] Symptoms experienced by these patients included nausea, acute colic, formation of a lead seam along the dental margin, peripheral neuropathy, loss of appetite and weight, as well as chronic fatigue and exhaustion.[54] An additional 597 cannabis users in the area of Leipzig reported for a screening program initiated by the local health office, of which 27% of patients were found to have levels exceeding human biomonitoring values (above 250 µg/L for men and 150 µg/L for premenopausal/fertile women), also necessitating treatment.[54] Lead contamination of cannabis products should be avoided and considered a major health risk to all users.
Mercury
Mercury is another hazardous heavy metal found in cannabis, silver in color, the only liquid metal at standard temperature (0° C or 273.15 K) and pressure (1 atm, 101.3kPa, or 760 mmHg) in its elemental form (Hg); however, it is also frequently found in organomercury compounds such as methylmercury, which is considered highly poisonous and deleterious to humans when ingested.[67] In this organomercury form, methylmercury bioaccumulates in the human body, and has a biological half-life of up to 80 days for methylmercury that does not cross the blood brain barrier.[68] However, after crossing the blood brain barrier, methylmercury persists for decades in the brain after the cessation of exposure.[69] Mercury has been found at up to 440 ng/g of dry mass of cannabis grown in volcanic soils in Hawaii.[10] This is cause for concern as smoking cannabis products greatly increases the risk of heavy metal toxicity, with mercury being absorbed 10 times more efficiently by the lungs than the gut.[10] Chronic human exposure to mercury vapor results in a variety of symptoms, largely neurological, including forgetfulness, irritability, restricted visual fields, tremors, and paranoia.[53] Considering the health risks, it is in the interest to all cannabis users that mercury contamination be avoided as it would have detrimental effects on all exposed.
Pesticide contamination
While many claim cannabis is naturally a pest-resistant crop[70][71], there is still abundant use of various types of pesticides to provide protection, including insecticides, fungicides, and plant growth regulators.[10][42] Unlike most crops that are grown in the United States, there are no federal guidelines provided by the Environmental Protection Agency (EPA) as to which pesticides or how much should be used on cannabis.[72] However, with the recent federal legalization of hemp in the U.S. under the 2016 and 2019 Farm Bills, the EPA has approved a limited number of pesticides for use with hemp-producing Cannabis plants in the U.S.[73] Previous to the release of this list, a lack of regulation led to the widespread use of hazardous pesticides, including bifenazate, myclobutanil, and daminozide, as well as several others intended for ornamental plants and which are not approved for human consumption.[10] For example, the pesticide contents of 26 cannabis samples obtained from Washington dispensaries were investigated, and 84% of the cannabis samples analyzed were found to contain up to 24 agents of insecticides, miticides, fungicides, insecticidal synergists, and plant growth regulators.[74] Also in 2016, it was found that 49% of cannabis samples obtained from California dispensaries contained pesticides that are purely for ornamental plants, including abamectin and bifenazate.[10] In 2016, it was also shown that Guardian pesticides, which were marketed as all-natural and containing only safe-to-consume chemicals like cinnamon oil and citric acid, did in fact contain abamectin.[10] Furthermore, it has been shown that 69% of the pesticides used in cultivation stay in cannabis during smoking and can create toxic pyrolytic side products, suggesting that pesticide-contaminated cannabis may pose a significant toxicological threat to its users.[75]
As many of these pesticides are lipophilic, they are soluble in the solvents used for extraction of cannabinoids, including CBD oils and other products using extracted cannabinoids. Naturally, this leads to concerns about contamination of cannabis with pesticides and the potential health risks that would accompany concentrating these pesticides in an extract. The authors provide examples of a few compounds found in cannabis from each class of pesticide and the potential health risks posed by each. While it is well beyond the scope of this paper to review all types of pesticides used to treat cannabis, it is clear that pesticides associated with cannabis and their individual health risks should be considered as important to growers and user alike. For the health of consumers, particularly those with compromised immune systems utilizing cannabis for its therapeutic properties, it is imperative that a standard protocol continue to be developed for the safe use and testing of pesticides in cannabis.
Insecticides
Bifenazate and abamectin are two commonly identified insecticides found on cannabis products that are known to be harmful to mammals.[76] Bifenazate, a spider miticide, is not considered to be acutely toxic, though it is considered to be toxic when chronically exposed to mammals.[77] In animal feeding studies, weight gain in males and weight loss in females were reported in response to chronic exposures of bifenazate in their diets.[78] Furthermore, bifenazate has only been approved for use on ornamental plants in the U.S., so the EPA has not released any information regarding human mutagenicity for this compound. Abamectin, a macrocyclic lactone, is generally considered safe with toxicity arising only after ingestion of large quantities and is approved for edible plants.[79] Although the exact mechanisms remain unclear, there is evidence that macrocyclic lactones in large doses may pass through the blood-brain barrier to produce γ-amino butyric acid -mimetic (GABA) toxicity-like effects.[79] Current gaps in the knowledge of the long-term effects of these compounds still exist, but cell culture and animal studies continue to shed new light on the overall health impacts of these compounds. Until these compounds are shown to be harmless when inhaled or ingested, their application to cannabis should be limited or ceased entirely to best protect the consumer's health.
Fungicides
Several fungicides have been reported in samples of cannabis all over the world, including known endocrine disruptors and hepatoxic compounds like imazalil and myclobutanil, respectively.[10][42] These fungicides are often found in higher concentrations in samples obtained from indoor grow facilities than outdoor operations.[10] As many medicinal cannabis cultivators are now using indoor facilities to cultivate hemp for CBD products year-round, this is a cause for concern in both the hemp- and drug-type markets.
Imazalil—also known as enilconazole—and myclobutanil have both been used to prevent fungal infection in cannabis.[10] Imazalil, a systemic fungicide used to control powdery mildew and other mold or fungal infections in crop plants, has been shown to be an androgen receptor agonist and endocrine disruptor in mammals.[80] It can cause detrimental mutations in genes controlling cholesterol metabolism and androgen conversion to estrogen that carry on and persist into the following generations in mammals.[81][82] Myclobutanil is an inhibitor of ergosterol production in fungus, which is essential for the formation of fungal cell walls.[83] In addition to being detrimental to fungi, myclobutanil can also inhibit cholesterol synthesis in mammals at high doses.[83][84] Neither of these compounds have been extensively studied in humans, but what is known about their effects on mammalian systems is cause for concern. These fungicides should not be considered safe to use for any cannabis cultivation, and their application should be avoided to protect the health of the consumer.
Plant growth regulators
Plant growth regulators are also commonly found in cannabis, including carcinogens and compounds that have been shown to be detrimental to mammals. Daminozide and paclobutraxol are two plant growth regulators pervasively found in cannabis. Daminozide is used to delay the ripening of fruits and is considered relatively nontoxic unless consumed at very high doses; however, it is still considered a human carcinogen by the EPA.[85] This may be a greater concern to the farmers and cultivators than the end user, although little is known about the chronic exposure to daminozide over long periods of time. Paclobutraxol is a plant growth retardant that inhibits the biosynthesis of the plant hormone gibberellin, which is responsible for shoot elongation.[86] Paclobutraxol has been shown to have detrimental effects on development in several aquatic species[87], and also to disrupt neurotransmitter levels in mice.[88] Considering little is known about the human health consequences of chronic exposure to plant growth regulators, the use of these compounds in cannabis cultivation should be regulated with the health of the consumer in mind.
Polycyclic aromatic hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental pollutants usually generated by the incomplete combustion of organic materials (e.g., oil, coal, and wood).[89] They are found in some CBD oils and may come either from uptake by the plant during growth or from contaminated carrier oils during product preparation.[90] Excessive PAH content in CBD oils can be attributed to the smoke from nearby forest fires or from drying cannabis with propane heaters.[91] Over 100 types of PAHs exist, and some of the most studied and well characterized (i.e., benzo anthracene, chrysene, benzo fluoranthene, benzo pyrene) are known to be hazardous carcinogens, and can found in cannabis products worldwide. In the E.U., 20 out of 29 tested CBD oil brands were shown to have PAH levels higher than the legislative limits of 20 mg/kg.[92] High levels of PAHs in CBD oil are likely to cause DNA methylation, DNA adducts, and alteration of histone methylation, which can lead to immunosuppression.[89] The elimination of PAHs in the environment is most studied in biological systems through multi-step metabolic pathways, primarily mixed-function oxidase systems, but they can degrade through oxidation reactions in the environment.[89] The extent to how any given PAH is eliminated is highly dependent on its unique physical and chemical properties.[89] While it may be impossible to eliminate PAHs in cannabis products due to the ubiquitous nature of PAHs in the environment and the risk of producing these compounds when smoking cannabis, consumer exposure can be reduced by addressing the sources of contamination and avoiding growing cannabis in heavily industrialized areas.[93]
Other foreign matter
Other debris such as metal fragments, hairs, dusts, machine oils, or insect parts can be found in some CBD oil products, as can be seen in other foods or food products.[11] The FDA considers these foreign contaminants a negligible health hazard, but clearly this needs to be addressed by manufactures to develop high-quality control standards required to limit and minimize any foreign matter contamination.
Discussion
With the recent legalization of cannabis in many states of the U.S., there have been several state regulatory commissions put into place that address the issue of quality control in terms of contaminants and the cannabinoid profile. However, cannabis testing requirements do vary from state to state in terms of the minimum number of contaminants that must be tested for, and only 15 states currently have a regulatory commission in place. For the safety and welfare of all users, both medicinal and recreational, there is a necessity for a standardized set of guidelines for cultivation and testing of cannabis products. There is currently only one set of guidelines—developed by the American Herbal Products Association—called Recommendations for Regulators — Cannabis Operations that provides a detailed set of recommended instructions on cultivation, packaging, testing, and dispensing of cannabis products (both THC and CBD products), which has proven invaluable for ensuring the safe cultivation of cannabis.[94] While these are a great set of guidelines, a more comprehensive understanding of the contamination of cannabis products is necessary to appropriately eliminate the possible deleterious health effects contaminates may cause. Unfortunately, the classification of cannabis as a Schedule I drug federally makes the development and implementation of nationwide standards impossible at the moment, which, if left unchanged, could lead to significant health complications in those turning to cannabis for its medicinal properties.
Acknowledgements
Author contributions
S-HP and BVH conceived the review idea. MC and ZM wrote the initial draft. S-HP and CP reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Institute of Cannabis Research (ICR).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- ↑ Chagas, M.H.N.; Zuardi, A.W.; Tumas, V. et al. (2014). "Effects of cannabidiol in the treatment of patients with Parkinson's disease: an exploratory double-blind trial". Journal of Psychopharmacology 28 (11): 1088–98. doi:10.1177/0269881114550355. PMID 25237116.
- ↑ McGuire, P.; Robson, P.; Cubala, W.J. et al. (2018). "Cannabidiol (CBD) as an Adjunctive Therapy in Schizophrenia: A Multicenter Randomized Controlled Trial". American Journal of Psychiatry 175 (3): 225–31. doi:10.1176/appi.ajp.2017.17030325. PMID 29241357.
- ↑ Jeong, S.; Yun, H.K.; Jeong, Y.A. et al. (2019). "Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells". Cancer Letters 447: 12–23. doi:10.1016/j.canlet.2019.01.011. PMID 30660647.
- ↑ Sharafi, G.; He, H.; Nikfarjam, M. (2019). "Potential Use of Cannabinoids for the Treatment of Pancreatic Cancer". Journal of Pancreatic Cancer 5 (1): 1–7. doi:10.1089/pancan.2018.0019. PMC PMC6352507. PMID 30706048. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6352507.
- ↑ 5.0 5.1 5.2 Marchetti, C. (2013). "Role of calcium channels in heavy metal toxicity". ISRN Toxicology 2013: 184360. doi:10.1155/2013/184360. PMC PMC3658387. PMID 23724297. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3658387.
- ↑ Kaplan, J.S.; Stella, N.; Catterall, W.A. et al. (2017). "Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome". Proceedings of the National Academy of Sciences of the United States of America 114 (42): 11229–234. doi:10.1073/pnas.1711351114. PMC PMC5651774. PMID 28973916. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5651774.
- ↑ Pauli, C.S.; Conroy, M.; Heuvel, B.D.V.. et al. (2020). "Cannabidiol Drugs Clinical Trial Outcomes and Adverse Effects". Frontiers in Pharmacology 11: 63. doi:10.3389/fphar.2020.00063. PMC PMC7053164. PMID 32161538. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7053164.
- ↑ Habibi, R.; Hoffman, S.J. (2018). "Legalizing cannabis violates the UN drug control treaties, but progressive countries like Canada have options". Ottawa Law Review 49 (2): 427–60. https://rdo-olr.org/en/2018/legalizing-cannabis-violates-the-un-drug-control-treaties-but-progressive-countries-like-canada-have-options/.
- ↑ Sarma, N.D.; Waye, A.; ElSholy, M.A. et al. (2020). "Cannabis Inflorescence for Medical Purposes: USP Considerations for Quality Attributes". Journal of Natural Products 83 (4): 1334–51. doi:10.1021/acs.jnatprod.9b01200. PMID 32281793.
- ↑ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 10.10 10.11 10.12 10.13 10.14 10.15 10.16 10.17 McPartland, J.M.; McKernan, K.J. (2017). "Contaminants of Concern in Cannabis: Microbes, Heavy Metals and Pesticides". In Chandra, S.; Lata, H.; ElSohly, M.A.. Cannabis sativa L. - Botany and Biotechnology. Springer International Publishing. pp. 457–74. doi:10.1007/978-3-319-54564-6. ISBN 9783319545646.
- ↑ 11.0 11.1 11.2 Dryburgh, L.M.; Bolan, N.S.; Grof, C.P.L. et al. (2018). "Cannabis contaminants: Sources, distribution, human toxicity and pharmacologic effects". British Journal of Clinical Pharmacology 84 (11): 2468-2476. doi:10.1111/bcp.13695. PMC PMC6177718. PMID 29953631. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6177718.
- ↑ Ruchlemer, R.; Amit-Kohn, M.; Raveh, D. et al. (2015). "Inhaled medicinal cannabis and the immunocompromised patient". Supportive Care in Cancer 23 (3): 819-22. doi:10.1007/s00520-014-2429-3. PMID 25216851.
- ↑ 13.0 13.1 Hazekamp, A. (2018). "The Trouble with CBD Oil". Medical Cannabis and Cannabinoids 1 (1): 65–72. doi:10.1159/000489287.
- ↑ Corroon, J.; MacKay, D.; Dolphin, W. (2020). "Labeling of Cannabidiol Products: A Public Health Perspective". Cannabis and Cannabinoid Research: 1–5. doi:10.1089/can.2019.0101.
- ↑ Gruber, S.A.; Dahlgren, M.K.; Sagar, K.A. et al. (2014). "Worth the wait: Effects of age of onset of marijuana use on white matter and impulsivity". Psychopharmacology 231 (8): 1455-65. doi:10.1007/s00213-013-3326-z. PMC PMC3967072. PMID 24190588. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3967072.
- ↑ Crean, R.D.; Crane, N.A.; Mason, B.J. (2011). "An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions". Journal of Addiction Medicine 5 (1): 1–8. doi:10.1097/ADM.0b013e31820c23fa. PMC PMC3037578. PMID 21321675. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3037578.
- ↑ Zamberletti, E.; Gabaglio, M.; Prini, P. et al. (2015). "Cortical neuroinflammation contributes to long-term cognitive dysfunctions following adolescent delta-9-tetrahydrocannabinol treatment in female rats". European Nueropsychopharmacology 25 (12): 2404–15. doi:10.1016/j.euroneuro.2015.09.021. PMID 26499171.
- ↑ Wang, G.S.; Roosevelt, G.; Heard, K. (2013). "Pediatric marijuana exposures in a medical marijuana state". JAMA Pediatrics 167 (7): 630–3. doi:10.1001/jamapediatrics.2013.140. PMID 23712626.
- ↑ Herod, L.; Gardner, B. (2018). "House Bill 18-1023" (PDF). State of Colorado. http://leg.colorado.gov/sites/default/files/documents/2018A/bills/2018a_1023_eng.pdf.
- ↑ Bonn-Miller, M.O.; Loflin. M.J.E.; Thomas, B.F. et al. (2017). "Labeling Accuracy of Cannabidiol Extracts Sold Online". JAMA 318 (17): 1708–9. doi:10.1001/jama.2017.11909. PMC PMC5818782. PMID 29114823. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5818782.
- ↑ Pavlovic, R.; Nenna, G.; Calvi, L. et al. (2018). "Quality Traits of "Cannabidiol Oils": Cannabinoids Content, Terpene Fingerprint and Oxidation Stability of European Commercially Available Preparations". Molecules 23 (5): 1230. doi:10.3390/molecules23051230. PMC PMC6100014. PMID 29783790. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6100014.
- ↑ Lachenmeier, D.W.; Habel, S.; Fischer, B. et al. (2019). "Are side effects of cannabidiol (CBD) products caused by tetrahydrocannabinol (THC) contamination?". F1000Research 8: 1394. doi:10.12688/f1000research.19931.3. PMC PMC7029751. PMID 32117565. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7029751.
- ↑ Gurley, B.J.; Murphy, T.P.; Gul, W. et al. (2020). "Content versus Label Claims in Cannabidiol (CBD)-Containing Products Obtained from Commercial Outlets in the State of Mississippi". Journal of Dietary Supplements 17 (5): 599–607. doi:10.1080/19390211.2020.1766634. PMID 32431186.
- ↑ Vandrey, R.; Raber, J.C.; Raber, M.E. et al. (2015). "Cannabinoid Dose and Label Accuracy in Edible Medical Cannabis Products". JAMA 313 (24): 2491–3. doi:10.1001/jama.2015.6613. PMID 26103034.
- ↑ 25.0 25.1 Blake, A.; Nahtigal, I. (2019). "The evolving landscape of cannabis edibles". Current Opinion in Food Science 28: 25–31. doi:10.1016/j.cofs.2019.03.009.
- ↑ Deiana, S. (2017). "Chapter 99 - Potential Medical Uses of Cannabigerol: A Brief Overview". In Preedy, V.R.. Handbook of Cannabis and Related Pathologies. Academic Press. pp. 958–67. doi:10.1016/B978-0-12-800756-3.00115-0. ISBN 9780128007563.
- ↑ 27.0 27.1 27.2 27.3 27.4 27.5 27.6 27.7 27.8 McKernan, K.; Spangler, J.; Helbert, Y. et al. (2016). "Metagenomic analysis of medicinal Cannabis samples; pathogenic bacteria, toxigenic fungi, and beneficial microbes grow in culture-based yeast and mold tests". F1000Research 5: 2471. doi:10.12688/f1000research.9662.1. PMC PMC5089129. PMID 27853518. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5089129.
- ↑ 28.0 28.1 28.2 28.3 McParltand, J.M.; Hillig, K.W. (2004). "Cannabis clinic Fusarium wilt". Journal of Industrial Hemp 9 (2): 67–77. doi:10.1300/J237v09n02_07.
- ↑ 29.0 29.1 29.2 Daley, P.; Lampach, D.; Sguerra, S. (12 September 2013). "Testing Cannabis for Contaminants" (PDF). BOTEC Analysis Corporation. https://lcb.wa.gov/publications/Marijuana/BOTEC%20reports/1a-Testing-for-Contaminants-Final-Revised.pdf.
- ↑ 30.0 30.1 Punja, Z.K.; Collyer, D.; Scott, C. et al. (2019). "Pathogens and Molds Affecting Production and Quality of Cannabis sativa L". Frontiers in Plant Science 10: 1120. doi:10.3389/fpls.2019.01120. PMC PMC6811654. PMID 31681341. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6811654.
- ↑ 31.0 31.1 31.2 Pitt, J.I.; Basílico, J.C.; Abarca, M.L. et al. (2000). "Mycotoxins and toxigenic fungi". Medical Mycology 38 (Suppl. 1): 41–6. doi:10.1080/mmy.38.s1.41.46. PMID 11204163.
- ↑ Marasas, W.F.O.; Riley, R.T.; Hendricks, K.A. et al. (2004). "Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: A potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize". Journal of Nutrition 134 (4): 711–6. doi:10.1093/jn/134.4.711. PMID 15051815.
- ↑ 33.0 33.1 Kamle, M.; Mahato, D.K.; Devi, S. et al. (2019). "Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies". Toxins 11 (6): 328. doi:10.3390/toxins11060328. PMC PMC6628439. PMID 31181628. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6628439.
- ↑ Gorai, S.; Saha, M.; Madhab, V. et al. (2019). "Talaromycosis (Penicilliosis): A Rare, Opportunistic Systemic Fungal Infection". Indian Journal of Dermatology 64 (4): 331–33. doi:10.4103/ijd.IJD_70_17. PMC PMC6714180. PMID 31516150. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6714180.
- ↑ Le, T.; Thanh, N.T.; Thwaites, G.E. (2020). "Talaromycosis (Penicilliosis)". In Ryan, E.T.; Hill, D.R.; Solomon, T. et al.. Hunter's Tropical Medicine and Emerging Infectious Diseases (10th ed.). Elsevier. pp. 682–5. ISBN 9780323555128.
- ↑ Gargani, Y.; Bishop, P.; Denning, D.W. (2011). "Too many mouldy joints - Marijuana and chronic pulmonary aspergillosis". Mediterranean Journal of Hematology and Infectious Diseases 3 (1): e2011005. doi:10.4084/MJHID.2011.005. PMC PMC3103256. PMID 21625309. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3103256.
- ↑ Sutton, S.; Lum, B.L.; Torti, F.M. (1986). "Possible risk of invasive pulmonary aspergillosis with marijuana use during chemotherapy for small cell lung cancer". Drug Intelligence and Clinical Pharmacy 20 (4): 289-91. doi:10.1177/106002808602000416. PMID 3009125.
- ↑ 38.0 38.1 38.2 Dehal, N.; Quimby, D. (2019). "Disseminated Fusariosis in a Patient With Acute Myeloid Leukemia: A Case Report". Cureus 11 (10): e5922. doi:10.7759/cureus.5922. PMC PMC6857921. PMID 31788380. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6857921.
- ↑ Sreeram, S.; Lobo, F.D.; Acharya, V. et al. (2017). "A Fortuitous Turn of Evidence in an Elderly Female - A Case of Pulmonary Fusariosis". Journal of Clinical and Diagnostic Research 11 (2): ED04–ED05. doi:10.7860/JCDR/2017/24736.9191. PMC PMC5376773. PMID 28384871. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5376773.
- ↑ McPartland, J.M.; Cubeta, M.A. (1997). "New species, combinations, host associations and location records of fungi associated with hemp (Cannabis sativa)". Mycological research 101 (7): 853-857. doi:10.1017/S0953756297003584.
- ↑ Martyny, J.W.; Serrano, K.a.; Schaeffer, J.W. et al. (2013). "Potential exposures associated with indoor marijuana growing operations". Journal of Occupational and Environmental Hygiene 10 (11): 622–39. doi:10.1080/15459624.2013.831986. PMID 24116667.
- ↑ 42.0 42.1 42.2 Sandler, L.N.; Beckerman, J.L.; Whitford, F. et al. (2019). "Cannabis as conundrum". Crop Protection 117: 37–44. doi:10.1016/j.cropro.2018.11.003.
- ↑ Winston, M.E.; Hampton-Marcell, J.; Zarraonaindia, I. et al. (2014). "Understanding cultivar-specificity and soil determinants of the cannabis microbiome". PLoS One 9 (6): e99641. doi:10.1371/journal.pone.0099641. PMC PMC4059704. PMID 24932479. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4059704.
- ↑ Scott, M.; Rani, M.; Samsatly, J. et al. (2018). "Endophytes of industrial hemp (Cannabis sativa L.) cultivars: identification of culturable bacteria and fungi in leaves, petioles, and seeds". Canadian Journal of Microbiology 64 (10): 664-680. doi:10.1139/cjm-2018-0108. PMID 29911410.
- ↑ Kim, K.S. (2015). "Human Meningitis-Associated Escherichia coli". EcoSal Plus 7 (1). doi:10.1128/ecosalplus.ESP-0015-2015. PMC PMC4881430. PMID 27223820. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4881430.
- ↑ Crofts, A.A.; Giovanetti, S.M.; Rubin, E.J. et al. (2018). "Enterotoxigenic E. coli virulence gene regulation in human infections". Proceedings of the National Academy of Sciences of the United States of America 115 (38): E8968-E8976. doi:10.1073/pnas.1808982115. PMC PMC6156659. PMID 30126994. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6156659.
- ↑ Valilis, E.; Ramsey, A.; Sidiq, S. et al. (2018). "Non-O157 Shiga toxin-producing Escherichia coli-A poorly appreciated enteric pathogen: Systematic review". International Journal of Infectious Diseases 76: 82–87. doi:10.1016/j.ijid.2018.09.002. PMID 30223088.
- ↑ Sobel, J. (2005). "Botulism". Clinical Infectious Diseases 41 (8): 1167–73. doi:10.1086/444507. PMID 16163636.
- ↑ McKernan, K.; Helbert, Y.; Ebling, H. et al. (2018). "Microbiological examination of nonsterile Cannabis products: Molecular Microbial Enumeration Tests and the limitation of Colony Forming Units". OSF Preprints. doi:10.31219/osf.io/vpxe5.
- ↑ Morgand, M.; Leclerq, A.; Maury, M.M. et al. (2018). "Listeria monocytogenes-associated respiratory infections: A study of 38 consecutive cases". Clinical Microbiology and Infection 24 (12): 1339.e1-1339.e5. doi:10.1016/j.cmi.2018.03.003. PMID 29549058.
- ↑ Bouwknegt, M.; Verhaelen, K.; Rzeżutka, A. et al. (2015). "Quantitative farm-to-fork risk assessment model for norovirus and hepatitis A virus in European leafy green vegetable and berry fruit supply chains". International Journal of Food Microbiology 198: 50–8. doi:10.1016/j.ijfoodmicro.2014.12.013. PMID 25598201.
- ↑ Pérez-Moreno, M.; Pérez-Lloret, P.; González-Soriano, J. et al. (2019). "Cannabis resin in the region of Madrid: Adulteration and contamination". Forensic Science International 298: 34–8. doi:10.1016/j.forsciint.2019.02.049. PMID 30878463.
- ↑ 53.0 53.1 Siegel, B.Z.; Garnier, L.; Siegel, S.M. (1988). "Mercury in Marijuana: Some of the problems arising from marijuana use might result from the intake of bioaccumulated mercury". BioScience 38 (9): 619–23. doi:10.2307/1310827.
- ↑ 54.0 54.1 54.2 54.3 Busse, F.P.; Fiedler, G.M.; Leichtle, A. et al. (2008). "Lead poisoning due to adulterated marijuana in Leipzig". Deutsches Arzteblatt International 105 (44): 757-62. doi:10.3238/arztebl.2008.0757. PMC PMC2696942. PMID 19623274. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2696942.
- ↑ Gauvin, D.V.; Zimmermann, Z.J.; Yoder, J. et al. (2018). "Marijuana toxicity: Heavy metal exposure through state-sponsored access to “la Fee Verte”". Pharmaceutical Regulatory Affairs 7 (1): 1000202. doi:10.4172/2167-7689.1000202.
- ↑ Vardhan, K.H.; Kumar, P.S.; Panda, R.C. (2019). "A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives". Journal of Molecular Liquids 290: 111197. doi:10.1016/j.molliq.2019.111197.
- ↑ 57.0 57.1 Pappas, R.S. (2011). "Toxic elements in tobacco and in cigarette smoke: Inflammation and sensitization". Metallomics 3 (11): 1181-98. doi:10.1039/c1mt00066g. PMC PMC4542087. PMID 21799956. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4542087.
- ↑ 58.0 58.1 Tellez-Plaza, M.; Jones, M.R.; Dominguez-Lucas, A. et al. (2013). "Cadmium exposure and clinical cardiovascular disease: A systematic review". Current Atherosclerosis Reports 15 (10): 356. doi:10.1007/s11883-013-0356-2. PMC PMC3858820. PMID 23955722. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3858820.
- ↑ 59.0 59.1 Singani, A.A.S.; Ahmadi, P. (2012). "Manure Application and Cannabis Cultivation Influence on Speciation of Lead and Cadmium by Selective Sequential Extraction". Soil and Sediment Contamintation 21 (3): 305–21. doi:10.1080/15320383.2012.664186.
- ↑ Browar, A.W.; Koufos, E.B.; Wei, Y. et al. (2018). "Cadmium Exposure Disrupts Periodontal Bone in Experimental Animals: Implications for Periodontal Disease in Humans". Toxics 6 (2): 32. doi:10.3390/toxics6020032. PMC PMC6027471. PMID 29899258. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6027471.
- ↑ Buha, A.; Wallace, D.; Matovic, V. et al. (2017). "Cadmium Exposure as a Putative Risk Factor for the Development of Pancreatic Cancer: Three Different Lines of Evidence". Biomedical Research International 2017: 1981837. doi:10.1155/2017/1981837. PMC PMC5733953. PMID 29349066. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5733953.
- ↑ Tinkov, A.A.; Filippini, T.; Ajsuvakova, O.P. et al. (2017). "The role of cadmium in obesity and diabetes". The Science of the Total Environment 601–602: 741–55. doi:10.1016/j.scitotenv.2017.05.224. PMID 28577409.
- ↑ Kasuya, M.; Teranishi, H.; Aishima, K. et al. (1992). "Water Pollution by Cadmium and the Onset of Itai-itai Disease". Water Science & Technology 25 (11): 149–56. doi:10.2166/wst.1992.0286.
- ↑ 64.0 64.1 Flora, G.; Gupta, D.; Tiwari, A. (2012). "Toxicity of lead: A review with recent updates". Interdisciplinary Toxicology 5 (2): 47–58. doi:10.2478/v10102-012-0009-2. PMC PMC3485653. PMID 23118587. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3485653.
- ↑ 65.0 65.1 65.2 Mason, L.H.; Harp, J.P.; Han, D.Y. (2014). "Pb neurotoxicity: Neuropsychological effects of lead toxicity". BioMed Research International 2014: 840547. doi:10.1155/2014/840547. PMC PMC3909981. PMID 24516855. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3909981.
- ↑ Entwistle, J.A.; Amaibi, P.M.; Dean, J.R. et al. (2019). "An apple a day? Assessing gardeners' lead exposure in urban agriculture sites to improve the derivation of soil assessment criteria". Environment International 122: 130–41. doi:10.1016/j.envint.2018.10.054. PMID 30449630.
- ↑ Hong, Y.-S.; Kim, Y.-M.; Lee, K.-E. (2012). "Methylmercury exposure and health effects". Journal of Preventive Medicine and Public Health 45 (6): 353–63. doi:10.3961/jpmph.2012.45.6.353. PMC PMC3514465. PMID 23230465. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3514465.
- ↑ Jo, S.; Woo, H.D.; Kwon, H.-J. et al. (2015). "Estimation of the Biological Half-Life of Methylmercury Using a Population Toxicokinetic Model". International Journal of Environmental Research and Public Health 12 (8): 9054–67. doi:10.3390/ijerph120809054. PMC PMC4555264. PMID 26264017. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4555264.
- ↑ Björkman, L.; Lundekvam, B.F.; Laegreid, T. et al. (2007). "Mercury in human brain, blood, muscle and toenails in relation to exposure: an autopsy study". Environmental Health 6: 30. doi:10.1186/1476-069X-6-30. PMC PMC2098763. PMID 17931423. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2098763.
- ↑ Park, S.-H.; Staples, S.K.; Gostin, E.L. et al. (2019). "Contrasting Roles of Cannabidiol as an Insecticide and Rescuing Agent for Ethanol–induced Death in the Tobacco Hornworm Manduca sexta". Scientific Reports 9: 10481. doi:10.1038/s41598-019-47017-7.
- ↑ McKernan, K.J.; Helbert, Y.; Kane, L.T. et al. (2020). "Sequence and annotation of 42 cannabis genomes reveals extensive copy number variation in cannabinoid synthesis and pathogen resistance genes". bioRxiv. doi:10.1101/2020.01.03.894428.
- ↑ Seltenrich, N. (2019). "Into the Weeds: Regulating Pesticides in Cannabis". Environmental Health Perspectives 127 (4): 042001. doi:10.1289/EHP5265.
- ↑ U.S. Environmental Protection Agency (December 2019). "Pesticide Products Registered for Use on Hemp". https://www.epa.gov/pesticide-registration/pesticide-products-registered-use-hemp. Retrieved 29 May 2020.
- ↑ Russo, E.B. (2016). "Current Therapeutic Cannabis Controversies and Clinical Trial Design Issues". Frontiers in Pharmacology 7: 309. doi:10.3389/fphar.2016.00309. PMC PMC5022003. PMID 27683558. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5022003.
- ↑ Sullivan, N.; Elzinga, S.; Raber, J.C. (2013). "Determination of Pesticide Residues in Cannabis Smoke". Journal of Toxicology 2013: 378168. doi:10.1155/2013/378168.
- ↑ Radi, A.M.; Mohammed, E.T.; Abushouk, A.I. et al. (2020). "The effects of abamectin on oxidative stress and gene expression in rat liver and brain tissues: Modulation by sesame oil and ascorbic acid". The Science of the Total Environment 701: 134882. doi:10.1016/j.scitotenv.2019.134882. PMID 31739238.
- ↑ European Food Safety Authority (2017). "Peer review of the pesticide risk assessment of the active substance bifenazate". EFSA Journal 15 (1): e04693. doi:10.2903/j.efsa.2017.4693. PMC PMC7009907. PMID 32625279. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7009907.
- ↑ Zarn, J.A.; O'Brien, C.D. (2018). "Current pesticide dietary risk assessment in light of comparable animal study NOAELs after chronic and short-termed exposure durations". Archives of Toxicology 92 (1): 157-167. doi:10.1007/s00204-017-2052-4. PMC PMC5773667. PMID 28929275. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5773667.
- ↑ 79.0 79.1 da Silva, W.A.M.; Guimarães, A.T.B.; Montalvão, M.F. et al. (2018). "The chronic exposure to abamectin causes spatial memory deficit and depressive behavior in mice". Chemosphere 194: 523–33. doi:10.1016/j.chemosphere.2017.12.028. PMID 29241126.
- ↑ Goetz, A.K.; Rockett, J.C.; Ren, H. et al. (2009). "Inhibition of rat and human steroidogenesis by triazole antifungals". Systems Biology in Reproductive Medicine 55 (5–6): 214–26. doi:10.3109/19396360903234045. PMID 19938956.
- ↑ Jin, C.; Luo, T.; Fu, Z. et al. (2018). "Chronic exposure of mice to low doses of imazalil induces hepatotoxicity at the physiological, biochemical, and transcriptomic levels". Environmental Toxicology 33 (6): 650-658. doi:10.1002/tox.22550. PMID 29451352.
- ↑ Jin, C.; Zhang, R.; Fu, Z. et al. (2019). "Maternal exposure to imazalil disrupts the endocrine system in F 1 generation mice". Molecular and Cellular Endocrinology 486: 105–12. doi:10.1016/j.mce.2019.03.002. PMID 30853599.
- ↑ 83.0 83.1 Hester, S.D.; Wolf, D.C.; Nesnow, S. et al. (2006). "Transcriptional profiles in liver from rats treated with tumorigenic and non-tumorigenic triazole conazole fungicides: Propiconazole, triadimefon, and myclobutanil". Toxicologic Pathology 34 (7): 879–94. doi:10.1080/01926230601047824. PMID 17178689.
- ↑ Berenstein, G.; Nasello, S.; Beiguel, E. et al. (2017). "Human and soil exposure during mechanical chlorpyrifos, myclobutanil and copper oxychloride application in a peach orchard in Argentina". The Science of the Total Environment 586: 1254–62. doi:10.1016/j.scitotenv.2017.02.129. PMID 28237465.
- ↑ Neff, R.A.; Goldman, L.R. (2005). "Regulatory parallels to Daubert: stakeholder influence, "sound science," and the delayed adoption of health-protective standards". American Journal of Public Health 95 (Suppl. 1): S81–91. doi:10.2105/AJPH.2004.044818. PMID 16030344.
- ↑ Rademacher, W. (2000). "Growth Retardants: Effects on Gibberellin Biosynthesis and Other Metabolic Pathways". Annual Review of Plant Physiology and Plant Molecular Biology 51: 501–31. doi:10.1146/annurev.arplant.51.1.501. PMID 15012200.
- ↑ Li, J.; Sun, L.; Zuo, Z. et al. (2012). "Exposure to paclobutrazol disrupts spermatogenesis in male Sebastiscus marmoratus". Aquatic Toxicology 122–23: 120–4. doi:10.1016/j.aquatox.2012.06.007. PMID 22789407.
- ↑ Xu, M.; Yang, F. (2020). "Integrated gender-related effects of profenofos and paclobutrazol on neurotransmitters in mouse". Ecotoxicology and Environmental Safety 190: 110085. doi:10.1016/j.ecoenv.2019.110085. PMID 31855789.
- ↑ 89.0 89.1 89.2 89.3 Abdel-Shafy, H.I.; Mansour, M.S.M. (2016). "A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation". Egyptian Journal of Petroleum 25 (1): 107–23. doi:10.1016/j.ejpe.2015.03.011.
- ↑ Večerka, J. (16 February 2018). "Warning for consumers of CBD and cannabis oils sold on the EU market". International Cannabis and Cannabinoids Institute. https://www.icci.science/en/article/news/warning-for-consumers-of-cbd-and-cannabis-oils-sold-on-the-eu-market/. Retrieved 22 January 2020.
- ↑ Zelinkova, Z.; Wenzl, T. (2015). "The Occurrence of 16 EPA PAHs in Food - A Review". Polycyclic Aromatic Compounds 35 (2–4): 248–84. doi:10.1080/10406638.2014.918550. PMC PMC4673601. PMID 26681897. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4673601.
- ↑ White, C.M. (2019). "A Review of Human Studies Assessing Cannabidiol's (CBD) Therapeutic Actions and Potential". Journal of Clinical Pharmacology 59 (7): 923–34. doi:10.1002/jcph.1387. PMID 30730563.
- ↑ Jett, J.; Stone, E.; Warren, G. et al. (2018). "Cannabis Use, Lung Cancer, and Related Issues". Journal of Thoracic Oncology 13 (4): 480–487. doi:10.1016/j.jtho.2017.12.013. PMID 29374567.
- ↑ American Herbal Products Association (2 February 2020). "Recommendations for Regulators - Cannabis Operations" (PDF). American Herbal Products Association. http://www.ahpa.org/Portals/0/PDFs/Committee/CC/Cannabis_Operations_Recommendations_Regulators.pdf.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. The original article lists references in alphabetical order; this version lists them in order of appearance, by design. A citation for Gorai et al. is found in the original references and in Table 1, but it is not included in-line in the original text; it has been inserted where it should presumably go for this version. The extremely long nomenclatures for bifenazate, abamectin, imazalil, and other pesticides/growth regulators that are included in the original article are omitted for this version for the sake of brevity, as they add little to the overall intent of the article.