Journal:Error evaluation in the laboratory testing process and laboratory information systems
| Full article title | Error evaluation in the laboratory testing process and laboratory information systems |
|---|---|
| Journal | Journal of Medical Biochemistry |
| Author(s) | Arifin, Azila; Yusof, Maryati |
| Author affiliation(s) | Universiti Kebangsaan Malaysia |
| Primary contact | Email: Maryati dot Yusof at ukm dot edu dot my |
| Year published | 2021 |
| Volume and issue | 40 |
| Page(s) | 1–11 |
| DOI | 10.5937/jomb0-31382 |
| ISSN | 1452-8258 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://aseestant.ceon.rs/index.php/jomb/article/view/31382 |
| Download | https://aseestant.ceon.rs/index.php/jomb/article/view/31382/17766 (PDF) |
Abstract
Background: The laboratory testing process consists of five analysis phases, featuring the total testing process (TTP) framework. Activities in laboratory processing, including those of testing, are error-prone and affect the use of laboratory information systems (LIS). This study seeks to identify error factors related to system use, as well as the first and last phases of the laboratory testing process, using a proposed framework known as the "total testing process for laboratory information systems" (TTP-LIS).
Methods: We conducted a qualitative case study evaluation in two private hospitals and a medical laboratory. We collected data using interviews, observations, and document analysis methods involving physicians, nurses, an information technology officer, and the laboratory staff. We employed the proposed framework and lean problem solving tools, namely value-stream mapping and the A3 process for data analysis.
Results: Errors in an LIS and the laboratory testing process were attributed to failure to fulfill user requirements, poor cooperation between the information technology unit and laboratory, inconsistency of software design in system integration, errors during inter-system data transmission, and lack of motivation in system use. The error factors are related to system development elements, namely latent failures that considerably affected information quality and system use. Errors in system development were also attributed to poor service quality.
Conclusions: Complex laboratory testing processes and LISs require rigorous evaluation in minimizing errors and ensuring patient safety. The proposed framework and lean approach are applicable for evaluating the laboratory testing process and LISs in a rigorous, comprehensive, and structured manner.
Keywords: case study, error, evaluation, framework, laboratory information system, lean, patient safety, total testing process, socio-technical
Introduction
A mistake or inefficiency in one of the stages of the laboratory testing chain can affect overall process implementation and management, and subsequently physician diagnosis.[1][2] However, a laboratory information system (LIS) is able to expedite and facilitate these and other interactions during the laboratory testing process.[3] Yet involvement of multiple units in testing workflow still requires effective use of the LIS to monitor task performance, ensure a smooth process, and readily identify errors. A complex, error prone, unreliable, and poorly designed LIS, on the other hand, may introduce errors identifiable in laboratory test results.[4][5] These outcomes can be further aggravated when the LIS links erroneous patient and test data to other units and institutions and involves data exchange because of complex intersystem interaction.[6] Further errors can also be introduced, attributable to human factors, including patient misidentification and erroneous test requests.[7]
To minimizes these errors, some labs have turned to the total testing process (TTP)[8], a unique framework that guides the testing process, and analyzes and minimizes testing error risk not only in the core laboratory but also in other clinical units.[7][9] The TTP includes internal and external laboratory activities that involve one or more procedures requiring staff interaction. However, the TTP should also address the role the LIS plays in the testing process.
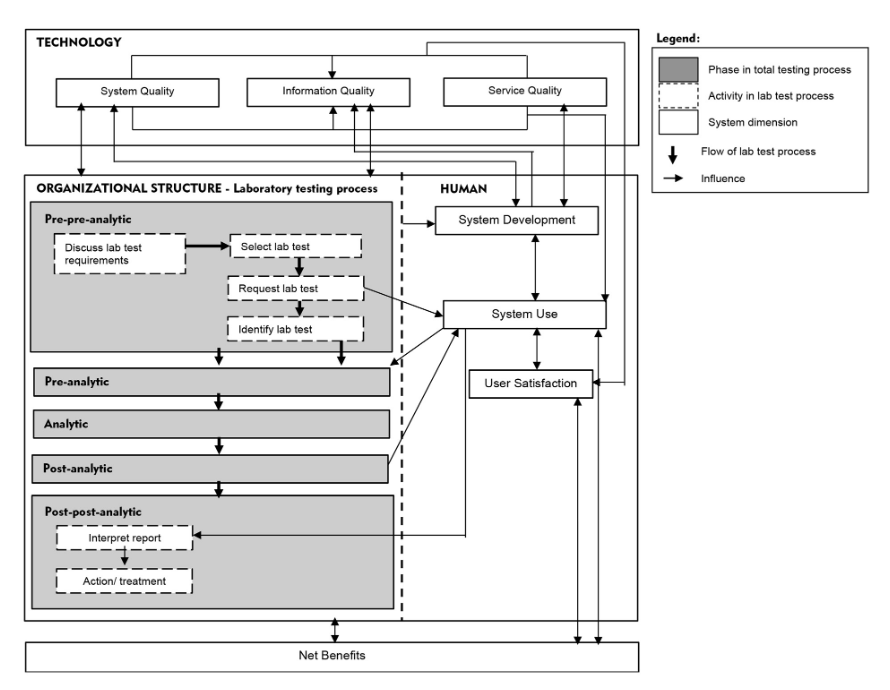
We propose a "total testing process for laboratory information systems" (TTP-LIS) framework on the basis of a combination of TTP and human, organization, technology, and fit (HOT-fit) frameworks.[10][11] The HOT aspects are crucial elements that complement the evaluation of the LIS and lab testing process. The proposed framework aims to illustrate a systematic, coordinated, and optimized laboratory testing process and LIS workflow to facilitate a rigorous error evaluation process.[12] The evaluation factors, dimensions, measures, and their relationships are depicted in Figure 1.
|
Error evaluation can benefit from lean, a quality improvement method that emphasizes removing process waste, including error. Various lean tools—such as value-stream mapping (VSM), 5 Why, and A3 problem-solving methods—have been widely used for process improvement.[13] A3 takes a structured approach to problem solving that uses a report tool to summarize the definition, scope, discovery process, findings, proposed action steps, and results from the problem analysis. A3 can be combined with other lean tools, such as VSM and 5 Why, to visualize and identify the root cause of problems. VSM is used to illustrate the overall process to identify waste and other problems and the appropriate solutions in the current and future state map, respectively. The problem can be scrutinized using the 5 Why tool to identify its root cause and mitigation strategy by asking a series of questions, either five times or any appropriate range. This study focused only on pre-pre-analytic and post-post-analytic phases of the TTP framework, given their high error rates[14][15], compared to other phases.
Material and methods
We conducted a subjectivist case study strategy employing qualitative methods in this summative evaluation to examine errors related to the LIS and the first and last phases of the lab testing process. A subjectivist approach enabled a comprehensive understanding of the healthcare context surrounding the management of LIS-induced error by generating detailed, insightful explanations.[16][17] We performed the evaluation by applying the TTP-LIS framework at two premier private hospitals in Malaysia. These cutting-edge hospitals have lead national health care efforts and are recognized by accreditation bodies such as the Malaysian Society for Quality in Health, Joint Commission International (XI), and Quality Management System (MS ISO 9001: 2015). The local Institutional Review Board deemed this study exempt from review. Author AA, a trained qualitative researcher, collected the data through interviews, non-participant observations, and document/artifact analysis methods.
Sampling
A purposeful snowball sampling method provided in-depth information from key informants. We identified participants from our initial contact with the lab director. We discussed the appropriateness of selected informants with the lab head based on the irrespective expertise, job scope, and abilities in providing the required information. Finally, we recruited 15 participants, including clinicians and management, lab, and IT staff (Table 1).
| ||||||||||||||||||
Data collection and analysis methods
The face-to-face, one-on-one interviews lasted for one to two hours for each informant who we queried on lab testing processes, LIS use, error and mistake incidents, their causes, and the strategies for mitigation and LIS improvement. We recorded (audio) and transcribed interviews. Observation took place in a medical lab for over a day on lab testing processes, from clinical requests to the production of lab results, to identify potential LIS-induced errors. We analyzed documents related to the LIS’ overall development, operation and management, process owner, backup system handling, and software and hardware management. We analyzed data thematically using the initial TTP-LIS evaluation framework.[12] In addition, we employed three lean tools—VSM, 5 Why, and A3—to visualize the current process, its problems, and root causes, as well as the desired (future) state of the first and last phases of lab testing.[13] We validated and refined the TTP framework with an expert who reviewed and acknowledged the said framework as a comprehensive evaluation tool for the lab testing process and LIS usage.
Results
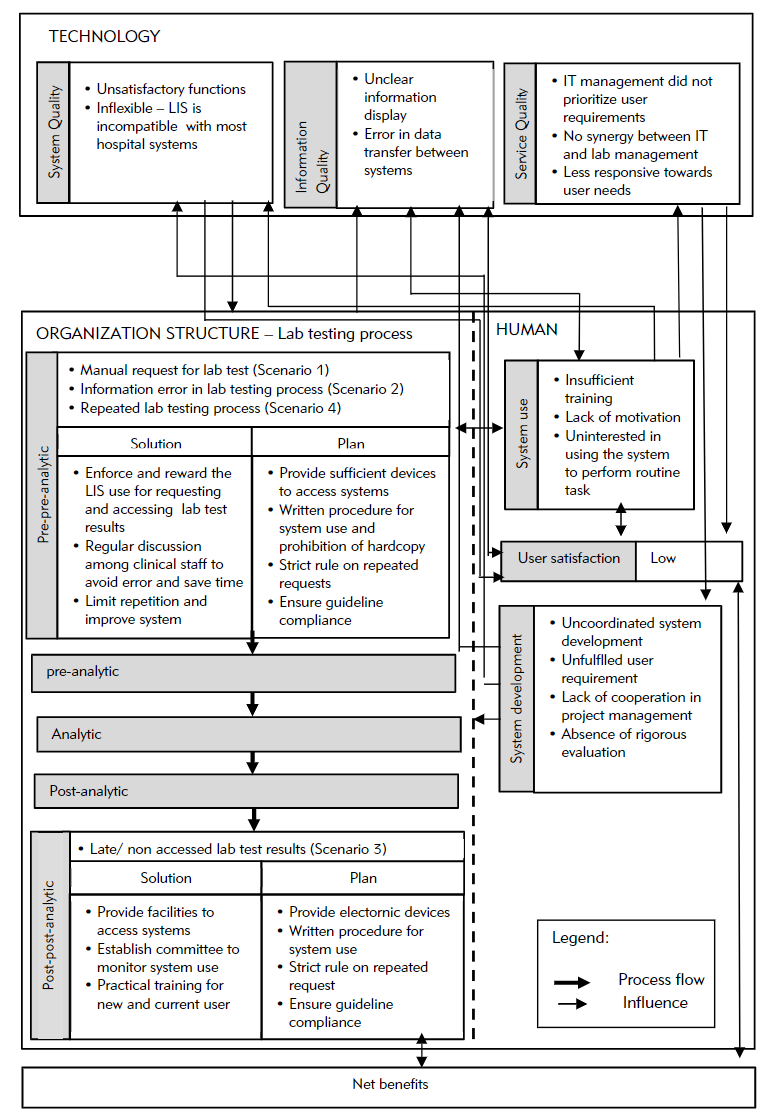
The hospitals PHA and PHB were established in the mid-1990s. They collaborated with a private laboratory, Lab C, which has managed most lab operations at all PH branches since 2000. The hospitals provide services to 3,000 to 4,000 patients at a time and provide educational services to medical and nursing students. Evaluation of the overall system used in the hospitals and laboratories involved the LIS, lab testing process, and other hospital information systems (HIS). The LIS evolved from a stand-alone system that only supports internal laboratory operations to a system with extended functions that are connected to other HISs. The LIS was also developed by the IT unit of Lab C, whereas the HIS was outsourced and operated by the hospital IT unit. Both systems are integrated in a new platform. The IT staff in Lab C provide training to LIS users. Figure 2 illustrates the overall findings according to the proposed TTP-LIS framework.
|
Human factors
Overall, the LIS was optimized by the lab staff, compared to the hospital staff. Many clinicians did not attend training because of time constraints and their heavy workload. Lack of training and exposure to an LIS resulted in low system use. Users, particularly senior physicians and nurses, were reluctant to use the LIS to request lab tests and access its results for various reasons, including "wasting time, [and] hassles to remember password, patient name, or ID" (Lab Head). According to a physician, "system use disrupted my task. Sometimes the LIS processes data slowly and requires time-consuming access, while the network is disrupted during lab test request. The manual form saved more time." A nurse stated that although "system use eased our task, our competency is low."
Broadly speaking, poor synergy and discrepancies between management and IT in planning and strategizing the LIS can affect system development and the subsequent non-optimized LIS use in clinical units. Poor system development is also attributed to poor service quality in terms of responsiveness, assurance (service providers’ skills, consideration and ability to provide trust and confidence[18]), and empathy from the service provider and hospital management.
For our study, LIS use was mandatory only in some PH branches, while others still operated manually. LIS development and use started from the laboratory and expanded to clinical units. Decisions for system development and use were made according to individual or other interests, including political issues such as conflict of interest and business profit, instead of system use. The integration of heterogeneous, outsourced, and in-house developed systems with different platforms, hardware, and software resulted in many system problems, such as unreadable information, unclear images (blurred, inappropriate pixel sizes, and display of system coding), and inaccessible information. These problems pose challenges to the clinical unit and the physicians’ decision making pertinent to patient diagnosis or treatment because of inaccurate data. Subsequently, these issues affect system use, user satisfaction, and the lab test process. Physicians and nurses preferred the manual method of requesting lab tests and obtaining lab test results, as they were perceived as being faster than using the LIS. Instead of increasing process efficiency, LIS use delayed tasks and disrupted the decision-making process. In short, system development outcomes significantly affect the system and information quality, and service quality determines the fulfillment of user requirements.
Technology factors
System quality influenced other factors, including system development, system use, the lab testing process, and user satisfaction. We identified errors that stemmed from poor LIS functions, including the a calculated total of lab test results that was less than the actual number of applied tests. Moreover "[some] lab test results accessed from LIS showed unexpected analysis when the results are linked to diagnosis results from the [clinical information system (CIS)]" (Dr. B).
Organizational factors
If there is no disruption, the whole lab testing process takes around 15–20 minutes. This includes affixing barcodes on specimen tubes and application forms, entering request information in the LIS, testing specimens, and verifying lab test results. We chose to analyze four process scenarios that were recommended by the informants according to their error impact on the overall workflow in terms of additional time, increased workload, material waste, and (most importantly) delay in patient treatment.
Scenario 1 (manual request of the lab test process and printing lab test results) became problematic as it resulted in extra workload for lab staff to routinely check or request missing information on the manual form or file and print documents. Additionally "the patient code on manual forms needs to be individually scanned and checked to ensure its consistency with the system" (lab head). Then, the lab test results must be printed and sent to physicians or nurses. Missing or lost results required another printout, and the same goes for physicians who requested patient lab test histories. Increased burden arose from the error chain, whereas a physician’s error rippled to the lab unit and the prescribing process that involves lab test results.
Erroneous test requests (Scenario 2) occurred for several reasons, as claimed by the informants. "We must perform the test upon receiving the sample and request form. We would not able to identify the request as a mistake when the request information is consistent with those of the system" (lab staff). "Choosing the wrong test commonly happened in critical situations where [the] physician does not have time to check [the] test requested by the nurse" and the nurse "forgets to verify it with the physician." A mistake is usually realized upon test completion.
Non-accessed/delayed lab test results (Scenario 3) recurred because of non-scrutinized processes or hasty decisions. According to the lab head, the situation affects staff efficiency, particularly when they must prioritize other urgent lab tests. Lab staff were puzzled when "a requested test's results were not accessed upon its completion, [thereby] indicating that the test is not needed, [a situation] which wasted our time and resources to conduct the test."
In Scenario 4, the repeated lab testing process is attributable to the inefficiency of the clinical unit and sample testing process. Lab testing was repeated when the laboratory or physician identified test results that were abnormal or fell outside the reference range lab test, or unidentified errors were present in the test request. Upon realizing these abnormalities and erroneous requests during results validation, the lab head ordered a second and correct test request, respectively. If the first and second test results were consistent, they were categorized as a critical case, and the physician was contacted immediately. Result abnormalities were entailed for the second test, whereas erroneous requests attributable to staff carelessness or inefficiency had to be avoided. Similar to Scenario 2, the prescriber’s verification is imperative before submitting the test request.
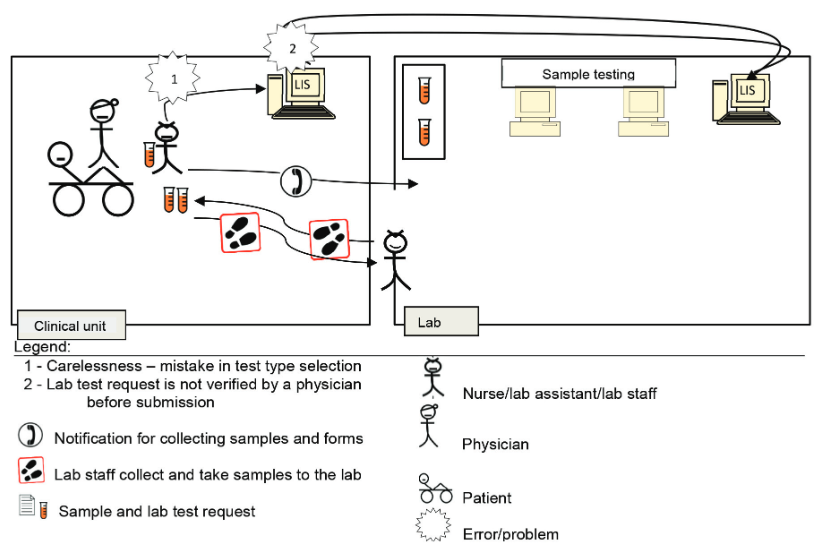
According to the four scenarios of the two lab testing processes for pre-pre-analysis and post-post-analysis (Figure 1), A3 diagrams were used to illustrate and elaborate upon the as-is and to-be processed elements, as demonstrated in Scenario 2 (Figure 2). The process was related to the lab test request by a nurse or clinical assistant using the LIS. A nurse was instructed by a physician to request a lab test using a CIS. The nurse labeled sample tubes and stored them while waiting for a lab staff member to collect them. Then, the nurse directly entered the related information for requesting the lab test in the computer unit. However, the test type that she chose differed from that desired by the physician.
Normally, neither the nurse nor the lab head would realize the mistake until the physician checks the order before submitting it to the LIS. Therefore, the test was processed normally according to the requested test type. Upon the test completion, the results were generated, checked, and verified by the lab head. Then, the results were submitted to the CIS via the LIS. A physician accessed the lab test results, only to realize that they are irrelevant. At this point, the charge was already forwarded to the finance unit for patient billing. This mistake required the physician to report the occurrence to the management and finance departments, and the charge would have to be paid by the hospital. Therefore, double-checking and verifying test requests are critical to avoid a chain of problems. The physician is responsible for rechecking requests, and the nurse must remind the physician about it before submission.
We illustrate these problems in the next subsection using an A3 report, to better aid in identifying the root cause and planning for mitigation.
A3 problem solving report for Scenario 2
Issue: Mistake in selecting lab test type during the request through the LIS.
Background: The nurse received instruction from the physician to request a lab test via the LIS. The nurse did not realize the she had mistakenly chosen the wrong test type during the request process.
Future state: The to-be processed flow diagram is similar to that of the as-is process (Figure 3), except for the replacement of the two problems with the following two solutions.
Solution steps: A detailed discussion occurred among the medical team concerning a mitigation plan to avoid recurring mistakes and resource waste.
|
Problem analysis:
| ||||||||||||||||
Implementation plan:
|
Costs/Benefits:
|
Discussion
We went through a relatively challenging, iterative process of constructing structured and comprehensive socio-technical factors in the TTP-LIS framework.[12] This study contributes to the existing knowledge by proposing a new framework based on the HOT-fit and TTP frameworks, as well as concepts in error management and process improvement, namely the lean methods. The TTP-LIS framework features a comprehensive evaluation method of socio-technical factors that can be applied flexibly in other processes and systems in a similar or different clinical settings. The findings showed the practicality of the TTP-LIS framework as an evaluation tool in identifying errors and their causal factors. The use of lean tools—namely, VSM, A3, and 5 Why—enabled us to analyze and visualize the root cause of problems in an objective and structured manner.[13][19] The evaluation of LIS-induced errors enabled the IT staff in both laboratory and hospitals to collaborate in improving LIS quality by synchronizing system development to reduce system integration problems and considering system functions according to user requirements. Human, lab testing process, organization, and technology factors are intertwined. Errors caused by humans[4][7], technology[5], and processes[3][9] disrupted the lab testing process workflow. Human factors mainly contributed to errors in the lab testing process and LIS, as proven in other studies.[7] Errors in system development and use that are attributed to human factors require continuous evaluation and monitoring to ensure quality. The LIS supports user needs[3][20] and routine tasks, and it reduces problems.[21] Mandatory use of the LIS among physicians and nurses is meant to increase the efficiency of routine tasks in the lab testing process. However, LIS use among clinicians is very low.
In general, the findings can be categorized in three ways: as a latent failure in system development, as poor error management, and as unsatisfactory lab testing processes and LIS use.
Latent failure in system development
System development highly contributed to error occurrence in LIS and HIS use in terms of the introduction of new technology, heterogeneous software, human–computer interaction, and communication issues within the system developer team. These factors are consistent with other findings.[3][5][6] These latent failures hinder the optimized potential of the LIS. In this case, the LIS developers really understood the requirements of the lab testing process and featured them as the main functions in the LIS. In contrast, the HIS was outsourced; the hospital management team identified more general user requirements. This resulted in integration conflict and subsequent errors, including unclear data requirements and inappropriate graph generation that affected physician decision making.
Latent failure is a major challenge for management and organizational decision makers. Strong collaboration between management with both hospital and laboratory units can aid in solving latent failure.[22] During system development, risk factors should also be considered apart from the cost. Heterogeneous system development methods increased error risk and cost. On the contrary, a unified system development method that considered user requirement reduced error risk. The study can be extended to further understand latent failure factors and identify optimum strategies to address them.
Poor error management
In general, LIS-induced errors require tackling the problems at their root cause and employing a basic solution method from the socio-technical perspectives, before quality improvement and automation[3][23][24][25][26], as proposed in our error management approach. Most identified errors can be mitigated through a joint, multi-discipline collaboration from all staff. However, monitoring is imperative at the outset[27] to ensure guideline compliance. An error management method serves as a tool to mitigate errors identified by the system or through routine error checking at the end of task completion. The absence of an error management system leads to recurring errors[28][29] that waste time, resources, and cost in terms of service or materials. Recurring errors also indicate ineffective and inefficient workflow and system use that negatively affects work motivation. Many error management strategies have been successfully proven in other industries and can be adopted in laboratory and clinical settings. These strategies include[19][30][31][32]:
- reducing cognitive load through automated records, notes, and processes (e.g., verification and checking);
- enhanced information access;
- imposing an error-proofing function for critical tasks such as preventing fatal drug instruction according to the dosage for certain patients;
- checking the error at its source (an individual process step);
- coordination of similar tasks; and
- minimizing individual involvement in a single task.
Lab testing processes and LIS use
User acceptance and sufficient training increase LIS use in lab testing workflow and subsequently ensure smooth flow and enhanced work quality.[3][7][21][33] However, a lean workflow is imperative prior to optimizing process automation to improve the core issues in the workflow itself.[3][13] Various efforts have been made to reduce errors in routine monitoring, particularly in the early and final phases of the lab testing process, given that both phases involve clinical instead of lab staff, who are more familiar with the related process. Therefore, inter-departmental cooperation is crucial for avoiding recurring errors.
In short, although all scenarios involved simple errors and mistakes, they posed various possible implications, such as inefficiency, high workload, adverse events, and patient safety issues. Inappropriate testing is not only wasteful and costly, but also risky to patients.[32] However, the processes can be streamlined and optimized through management and mitigation of processes and errors. Automated interventions such as an ordering system that alerts prescribers can educate them about requesting inappropriate or repeated testing.[32] Moreover, autoverification is widely reported to have potential for facilitating safe, efficient, and reliable tools.[31][34]
We propose a comprehensive plan to avoid errors in the early and final lab testing process. The steps include:
- analyzing and redesigning workflow according to lean methods;
- establishing clear written and digital procedures;
- improving system training for users;
- outlining indicators for quality monitoring; and
- improving communication and synergy among healthcare and laboratory professionals.
The procedure for lab testing workflow must clarify identifying patients; gathering, labeling, and transferring specimens; and preparing analyses. The responsible individual must understand and acknowledge the procedure and its importance, the potential risks, and the potential effects on the sample and, subsequently, to the patient because of procedure noncompliance. All steps require ongoing training and efficient assessment.
Study limitations
The short duration of the observations limited the detailed evaluation of possible error incidents during the lab test process, but this situation was offset with a briefing from the lab head. Moreover, documents related to the LIS' use and the lab testing processes are restricted as they are regarded as private and confidential. Furthermore, manual requests for laboratory tests limit the evaluation of LIS use in clinical units, particularly in the pre-pre-analysis phase. Nevertheless, the rich interview data compensate for this constraint.
Abbreviations
CIS: clinical information system
HIS: hospital information system
HOT-fit: human, organization, technology, and fit framework
LIS: laboratory information system
TTP: total testing process
TTP-LIS: total testing process for laboratory information system
VSM : value-stream mapping
Acknowledgements
Author contributions
All authors accept responsibility for the entire content of this manuscript and have approved its submission.
Funding
This work was supported by the Ministry of Higher Learning Malaysia (Grant no.s FRGS/1/2018/ICT04/UKM/02/5 and ERGS/1/2011/STG/UKM/02/46).
Informed consent
We obtained informed consent from all individuals involved in this study.
Conflicts of interest
All the authors declare that they have no conflict of interest in this work.
References
- ↑ Dighe, AS; Baron, JM (2011). "Computerized provider order entry in the clinical laboratory" (in en). Journal of Pathology Informatics 2 (1): 35. doi:10.4103/2153-3539.83740. ISSN 2153-3539. PMC PMC3162747. PMID 21886891. http://www.jpathinformatics.org/text.asp?2011/2/1/35/83740.
- ↑ Carraro, Paolo; Zago, Tatiana; Plebani, Mario (1 March 2012). "Exploring the Initial Steps of the Testing Process: Frequency and Nature of Pre-Preanalytic Errors" (in en). Clinical Chemistry 58 (3): 638–642. doi:10.1373/clinchem.2011.175711. ISSN 0009-9147. https://academic.oup.com/clinchem/article/58/3/638/5620542.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Yeo, Cp; Ng, Wy (1 November 2018). "Automation and productivity in the clinical laboratory: experience of a tertiary healthcare facility". Singapore Medical Journal: 597–601. doi:10.11622/smedj.2018136. PMC PMC6250760. PMID 30498842. http://www.smj.org.sg/article/automation-and-productivity-clinical-laboratory-experience-tertiary-healthcare-facility.
- ↑ 4.0 4.1 Bowie, Paul; Price, Julie; Hepworth, Neil; Dinwoodie, Mark; McKay, John (1 November 2015). "System hazards in managing laboratory test requests and results in primary care: medical protection database analysis and conceptual model" (in en). BMJ Open 5 (11): e008968. doi:10.1136/bmjopen-2015-008968. ISSN 2044-6055. PMC PMC4663465. PMID 26614621. https://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2015-008968.
- ↑ 5.0 5.1 5.2 Mathews, Althea; Marc, David (2017). "Usability evaluation of laboratory information systems" (in en). Journal of Pathology Informatics 8: 40. doi:10.4103/jpi.jpi_24_17. ISSN 2153-3539. PMC PMC5653961. PMID 29114434. http://www.jpathinformatics.org/text.asp?2017/8/1/40/215895.
- ↑ 6.0 6.1 Rajamani, Sripriya; Kayser, Ann; Emerson, Emily; Solarz, Sarah (21 September 2018). "Evaluation of Data Exchange Process for Interoperability and Impact on Electronic Laboratory Reporting Quality to a State Public Health Agency". Online Journal of Public Health Informatics 10 (2): e204. doi:10.5210/ojphi.v10i2.9317. ISSN 1947-2579. PMC PMC6194099. PMID 30349622. https://journals.uic.edu/ojs/index.php/ojphi/article/view/9317.
- ↑ 7.0 7.1 7.2 7.3 7.4 Sonmez, Cigdem; Yıldız, Ummugulsum; Akkaya, Nedim; Taneli, Fatma (20 March 2020). "Preanalytical Phase Errors: Experience of a Central Laboratory" (in en). Cureus 12 (3): e7335. doi:10.7759/cureus.7335. ISSN 2168-8184. PMC PMC7164707. PMID 32313776. https://www.cureus.com/articles/28897-preanalytical-phase-errors-experience-of-a-central-laboratory.
- ↑ Lundberg, G.D (1 February 1999). "How clinicians should use the diagnostic laboratory in a changing medical world" (in en). Clinica Chimica Acta 280 (1-2): 3–11. doi:10.1016/S0009-8981(98)00193-4. https://linkinghub.elsevier.com/retrieve/pii/S0009898198001934.
- ↑ 9.0 9.1 Plebani, Mario; Piva, Elisa (1 October 2010). "Medical Errors: Pre-Analytical Issue in Patient Safety". Journal of Medical Biochemistry 29 (4): 310–314. doi:10.2478/v10011-010-0039-2. ISSN 1452-8266. https://scindeks.ceon.rs/article.aspx?artid=1452-82581004310P.
- ↑ Yusof, Maryati Mohd.; Kuljis, Jasna; Papazafeiropoulou, Anastasia; Stergioulas, Lampros K. (1 June 2008). "An evaluation framework for Health Information Systems: human, organization and technology-fit factors (HOT-fit)" (in en). International Journal of Medical Informatics 77 (6): 386–398. doi:10.1016/j.ijmedinf.2007.08.011. https://linkinghub.elsevier.com/retrieve/pii/S1386505607001608.
- ↑ Yusof, Maryati Mohd. (1 July 2015). "A case study evaluation of a Critical Care Information System adoption using the socio-technical and fit approach" (in en). International Journal of Medical Informatics 84 (7): 486–499. doi:10.1016/j.ijmedinf.2015.03.001. https://linkinghub.elsevier.com/retrieve/pii/S1386505615000611.
- ↑ 12.0 12.1 12.2 Yusof, Maryati M.; Arifin, Azila (1 November 2016). "Towards an evaluation framework for Laboratory Information Systems" (in en). Journal of Infection and Public Health 9 (6): 766–773. doi:10.1016/j.jiph.2016.08.014. https://linkinghub.elsevier.com/retrieve/pii/S1876034116301344.
- ↑ 13.0 13.1 13.2 13.3 Jimmerson, Cindy LeDuc (2010). Value stream mapping for healthcare made easy. Boca Raton: CRC Press. ISBN 978-1-4200-7852-7. OCLC 301879954. https://www.worldcat.org/title/mediawiki/oclc/301879954.
- ↑ Carraro, Paolo; Plebani, Mario (1 July 2007). "Errors in a Stat Laboratory: Types and Frequencies 10 Years Later" (in en). Clinical Chemistry 53 (7): 1338–1342. doi:10.1373/clinchem.2007.088344. ISSN 0009-9147. https://academic.oup.com/clinchem/article/53/7/1338/5627526.
- ↑ Lippi, Giuseppe; Betsou, Fay; Cadamuro, Janne; Cornes, Michael; Fleischhacker, Michael; Fruekilde, Palle; Neumaier, Michael; Nybo, Mads et al. (26 June 2019). "Preanalytical challenges – time for solutions" (in en). Clinical Chemistry and Laboratory Medicine (CCLM) 57 (7): 974–981. doi:10.1515/cclm-2018-1334. ISSN 1437-4331. https://www.degruyter.com/document/doi/10.1515/cclm-2018-1334/html.
- ↑ Friedman, Charles P.; Wyatt, J. (2006). Evaluation methods in biomedical informatics. Health informatics (2nd ed ed.). New York: Springer. ISBN 978-0-387-25889-8.
- ↑ Yin, Robert K. (2018). Case study research and applications: design and methods (Sixth edition ed.). Los Angeles: SAGE. ISBN 978-1-5063-3616-9.
- ↑ Pitt, Leyland F.; Watson, Richard T.; Kavan, C. Bruce (1 June 1995). "Service Quality: A Measure of Information Systems Effectiveness". MIS Quarterly 19 (2): 173-187. doi:10.2307/249687. https://www.jstor.org/stable/249687?origin=crossref.
- ↑ 19.0 19.1 Jimmerson, Cindy; Weber, Dorothy; Sobek, Durward K. (1 May 2005). "Reducing Waste and Errors: Piloting Lean Principles at Intermountain Healthcare" (in en). The Joint Commission Journal on Quality and Patient Safety 31 (5): 249–257. doi:10.1016/S1553-7250(05)31032-4. https://linkinghub.elsevier.com/retrieve/pii/S1553725005310324.
- ↑ Croxatto, A.; Prod'hom, G.; Faverjon, F.; Rochais, Y.; Greub, G. (1 March 2016). "Laboratory automation in clinical bacteriology: what system to choose?" (in en). Clinical Microbiology and Infection 22 (3): 217–235. doi:10.1016/j.cmi.2015.09.030. https://linkinghub.elsevier.com/retrieve/pii/S1198743X16000069.
- ↑ 21.0 21.1 McGrowder, D.; Bishop R. (2015). "An Evaluation of Laboratory Information Systems in Medical Laboratories in Jamaica". In Moumtzoglou, Anastasius; Kastania, Anastasia; Archondakis, Stavros. Laboratory Management Information Systems: Current Requirements and Future Perspectives. Advances in Healthcare Information Systems and Administration. IGI Global. pp. 280–296. doi:10.4018/978-1-4666-6320-6.ch014. ISBN 978-1-4666-6320-6. http://services.igi-global.com/resolvedoi/resolve.aspx?doi=10.4018/978-1-4666-6320-6.
- ↑ Fondahn, Emily; Lane, Michael; Vannucci, Andrea, eds. (2016). "Introduction to Patient Safety". The Washington manual of patient safety and quality improvement. Philadelphia: Wolters Kluwer. pp. 115–22. ISBN 978-1-4511-9355-8.
- ↑ Ellwood, P. M.; Lundberg, G. D. (2 October 1996). "Managed care: a work in progress". JAMA 276 (13): 1083–1086. ISSN 0098-7484. PMID 8847772. https://pubmed.ncbi.nlm.nih.gov/8847772.
- ↑ Hawkins, Robert (1 January 2012). "Managing the Pre- and Post-analytical Phases of the Total Testing Process" (in en). Annals of Laboratory Medicine 32 (1): 5–16. doi:10.3343/alm.2012.32.1.5. ISSN 2234-3806. PMC PMC3255486. PMID 22259773. http://annlabmed.org/journal/view.html?doi=10.3343/alm.2012.32.1.5.
- ↑ Yusof, Maryati Mohd (2019). "A Socio-Technical and Lean Approach Towards a Framework for Health Information Systems-Induced Error". Studies in Health Technology and Informatics 257: 508–512. ISSN 1879-8365. PMID 30741248. https://pubmed.ncbi.nlm.nih.gov/30741248.
- ↑ Mohd. Yusof, Maryati; Takeda, Toshihiro; Mihara, Naoki; Matsumura, Yasushi (2020). "Process Approach for Managing Health Information System-Induced Medication Errors". Digital Personalized Health and Medicine: 1036–1040. doi:10.3233/SHTI200319. https://ebooks.iospress.nl/doi/10.3233/SHTI200319.
- ↑ Becher, Elise C.; Chassin, Mark R. (1 May 2001). "Improving Quality, Minimizing Error: Making It Happen" (in en). Health Affairs 20 (3): 68–81. doi:10.1377/hlthaff.20.3.68. ISSN 0278-2715. http://www.healthaffairs.org/doi/10.1377/hlthaff.20.3.68.
- ↑ Deetz, Carl O.; Nolan, Debra K.; Scott, Mitchell G. (1 January 2012). "An Examination of the Usefulness of Repeat Testing Practices in a Large Hospital Clinical Chemistry Laboratory" (in en). American Journal of Clinical Pathology 137 (1): 20–25. doi:10.1309/AJCPWPBF62YGEFOR. ISSN 0002-9173. https://academic.oup.com/ajcp/article-lookup/doi/10.1309/AJCPWPBF62YGEFOR.
- ↑ Kwok, J (1 May 2005). "Unnecessary repeat requesting of tests: an audit in a government hospital immunology laboratory" (in en). Journal of Clinical Pathology 58 (5): 457–462. doi:10.1136/jcp.2004.021691. ISSN 0021-9746. PMC PMC1770647. PMID 15858114. http://jcp.bmj.com/cgi/doi/10.1136/jcp.2004.021691.
- ↑ Spath, Patrice L., ed. (2011). Error reduction in health care: a systems approach to improving patient safety (Second Edition ed.). San Francisco: Jossey-Bass. ISBN 978-0-470-50240-2.
- ↑ 31.0 31.1 Randell, Edward W.; Yenice, Sedef; Khine Wamono, Aye Aye; Orth, Matthias (1 November 2019). "Autoverification of test results in the core clinical laboratory" (in en). Clinical Biochemistry 73: 11–25. doi:10.1016/j.clinbiochem.2019.08.002. https://linkinghub.elsevier.com/retrieve/pii/S0009912019306630.
- ↑ 32.0 32.1 32.2 Ambaraghassi, Georges; Béliveau, Claire; Labbé, Annie-Claude; Lavallée, Christian (1 February 2019). "Relevance or performance: potential savings associated with verification of prior results before performing microbiology analysis" (in en). Diagnostic Microbiology and Infectious Disease 93 (2): 136–139. doi:10.1016/j.diagmicrobio.2018.09.009. https://linkinghub.elsevier.com/retrieve/pii/S0732889318303833.
- ↑ Khajouei, R.; Saghaeiannejad, S.; Jahanbakhsh, M. et al. (2015). "Assessment of the Performance of the Laboratory Information System (LIS) Based on the Standards of the American National Standards Institute (ANSI)". Journal of Health and Biomedical Informatics 2 (1): 8–16. http://jhbmi.ir/article-1-80-en.html.
- ↑ Wang, Zhongqing; Peng, Cheng; Kang, Hui; Fan, Xia; Mu, Runqing; Zhou, Liping; He, Miao; Qu, Bo (1 December 2019). "Design and evaluation of a LIS-based autoverification system for coagulation assays in a core clinical laboratory" (in en). BMC Medical Informatics and Decision Making 19 (1): 123. doi:10.1186/s12911-019-0848-2. ISSN 1472-6947. PMC PMC6609390. PMID 31269951. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-019-0848-2.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation, spelling, and grammar. In some cases important information was missing from the references, and that information was added. A few citations in the original skip ahead and are not in numerical order; LIMSwiki sorts citations by order of appearance, and thus the citation ordering is slightly different than the original.