Journal:Generating big data sets from knowledge-based decision support systems to pursue value-based healthcare
| Full article title | Generating big data sets from knowledge-based decision support systems to pursue value-based healthcare |
|---|---|
| Journal | International Journal of Interactive Multimedia and Artificial Intelligence |
| Author(s) | González-Ferrer, Arturo; Seara, Germán; Cháfer, Joan; Mayol, Julio |
| Author affiliation(s) | Instituto de Investigación Sanitaria San Carlos, Hospital Universitario Clínico San Carlos |
| Primary contact | Email: arturogf at gmail dot com |
| Year published | 2018 |
| Volume and issue | 4 (7) |
| Page(s) | 42–46 |
| DOI | 10.9781/ijimai.2017.03.006 |
| ISSN | 1989-1660 |
| Distribution license | Creative Commons Attribution 3.0 Unported |
| Website | http://www.ijimai.org/journal/node/1626 |
| Download | http://www.ijimai.org/journal/sites/default/files/files/2017/03/ijimai_4_7_6_pdf_12585.pdf (PDF) |
|
|
This article should not be considered complete until this message box has been removed. This is a work in progress. |
Abstract
Talking about big data in healthcare we usually refer to how to use data collected from current electronic medical records, either structured or unstructured, to answer clinically relevant questions. This operation is typically carried out by means of analytics tools (e.g., machine learning) or by extracting relevant data from patient summaries through natural language processing techniques. From other perspectives of research in medical informatics, powerful initiatives have emerged to help physicians make decisions, in both diagnostics and therapeutics, built from existing medical evidence (i.e., knowledge-based decision support systems). Many of the problems these tools have shown, when used in real clinical settings, are related to their implementation and deployment, more than failing in their support; however, technology is slowly overcoming interoperability and integration issues. Beyond the point-of-care decision support these tools can provide, the data generated when using them, even in controlled trials, could be used to further analyze facts that are traditionally ignored in the current clinical practice. In this paper, we reflect on the technologies available to make the leap and how they could help drive healthcare organizations shifting to a value-based healthcare philosophy.
Keywords: big data, DSS, e-health, knowledge management, management systems
Introduction
Healthcare made a big step towards modernization with the emergence of the evidence-based medicine (EBM) concept in the late 1980s.[1] EBM is an approach to medical practice that aims to apply the best known scientific evidence into clinical decision-making regarding diagnosis and effective management of specific conditions and diseases. While the EBM concept was generally well received by care professionals, many factors — such as their daily work conditions or their high work load — affect putting into practice this approach in the expected way. A recent report from the Institute of Medicine in 2012 revealed that only 10 to 20 percent of the decisions clinicians make are evidence-based.[2] This fact reflects the need for medical practitioners, supported by their healthcare organizations, to make a shift in their behavior about the way clinical practice is currently carried out.
The idea of EBM emerged in very different conditions from the current scenario. An explosion of technical possibilities — in nearly thirty years — have come into place to help organizations take a more modern approach, providing them with support in this regard. Not only epidemiological research can drive EBM, but also new data-oriented approaches. When saying “data-oriented,” we refer to data about the real daily clinical practice: how, when, why, and by whom are clinical actions carried out (or not), and what are the health results of those actions. Nonetheless, this might still be hampered by the current design of electronic medical records (EMRs) and by the role and focus that contemporary doctors should adopt. The use of EMRs by physicians could be insufficient, as recognized by studies[2] that expose that, even after post-digitalization of healthcare, they are not utilized to their maximum potential at all.
The fact that the EBM approach was crafted with the goal in mind of pursuing effectiveness in disease management left behind the consideration of organizational and human factors that are crucial in how decisions are truly made. By analyzing data generated by healthcare organizations we could yield information concerning the pitfalls that are hindering evidence-based clinical actions. At the same time, new evidence could be unveiled that is probably not considered in the current production of clinical practice guidelines (CPGs). For example, Toussi et al.[3] used data mining techniques to find out how physicians prescribe medications in diverse cases with various clinical conditions, in order to complement existing clinical guidelines where absence of enough evidence occur. Furthermore, specific training actions could be directed to address common failures detected in the management of medical conditions.
Therefore, the problem that healthcare organizations are trying to solve, under the hypothesis that the “big data” paradigm will change the way clinical practice is currently carried out, is how they can produce data that help to unveil real clinical behavior and "mindlines,"[4] linked with other organizational data (e.g., costs) and context information that could be behind their actions and decisions. Only making this analysis possible will they be able to change their philosophy to pursue and underpin value, beyond so-called effectiveness. And value here means detecting which actions — later possibly abstracted into policies — could really improve the behavior of the organizations and care professionals for the better care of their patients.
In this paper, we intend to reflect on some existing techniques beyond current electronic medical records (EMRs) that can help to generate such data sets, as well as considerations to be made, providing some examples of initiatives we are trying to push forward from the Innovation Unit of Hospital Universitario Clínico San Carlos (HUCSC).
Knowledge-based decision support systems (KB-DSS)
In 2007, Gartner[5] reported a five-stage evolution model for electronic health records (EHRs) where they established a path of characteristics, in terms of eight core capabilities, that EHRs should follow in order to provide the proper support to care professionals. Systems complying with Generation 3 requisites were supposed to be able to bring evidence-based medicine to the point of care, and theoretically coincide with the capabilities of most available EHRs, which progressed mainly through the core capabilities of system management, interoperability, and clinical data models, though there is still space for improvement today. Generation 4 was expected to improve the core capabilities of decision support, clinically relevant data analysis, presentation, and clinical workflow management.
More recently, Greenes offered his view about the past and future of knowledge-driven health IT[6], stating that current EHR systems were built for a model that is now old and even inappropriate, supported by proprietary infrastructures and knowledge content. He also mentioned the gradual increase in knowledge-based applications during the 2000s, with the creation of computer-interpretable clinical guideline formalisms like GLIF[7] and others.[8] By that time, these systems were having little penetration into real clinical settings, mostly due to the lack of pervasiveness of standards and the use of proprietary tools. Fortunately, this fact is something widely recognized by the current health IT community, and steps have been taken to tackle these problems. From requirements analysis of data standards[9] and development data integration mechanisms[10] for making DSS interoperable, the emergence of new lightweight web services standards like the HL7 Fast Healthcare Interoperability Resources (FHIR)[11], to substantial investments from public bodies that ended up with real deployments and piloting of patient guidance systems. A good example is MobiGuide[10][12], a project funded by the European Commission under the seventh framework program (FP7). Its goal was to create an intelligent KB-DSS to help physicians and patients taking the most appropriate decisions to manage concrete conditions (atrial fibrillation, gestational diabetes) using a back-end server and wearable sensors to monitor patients' status.
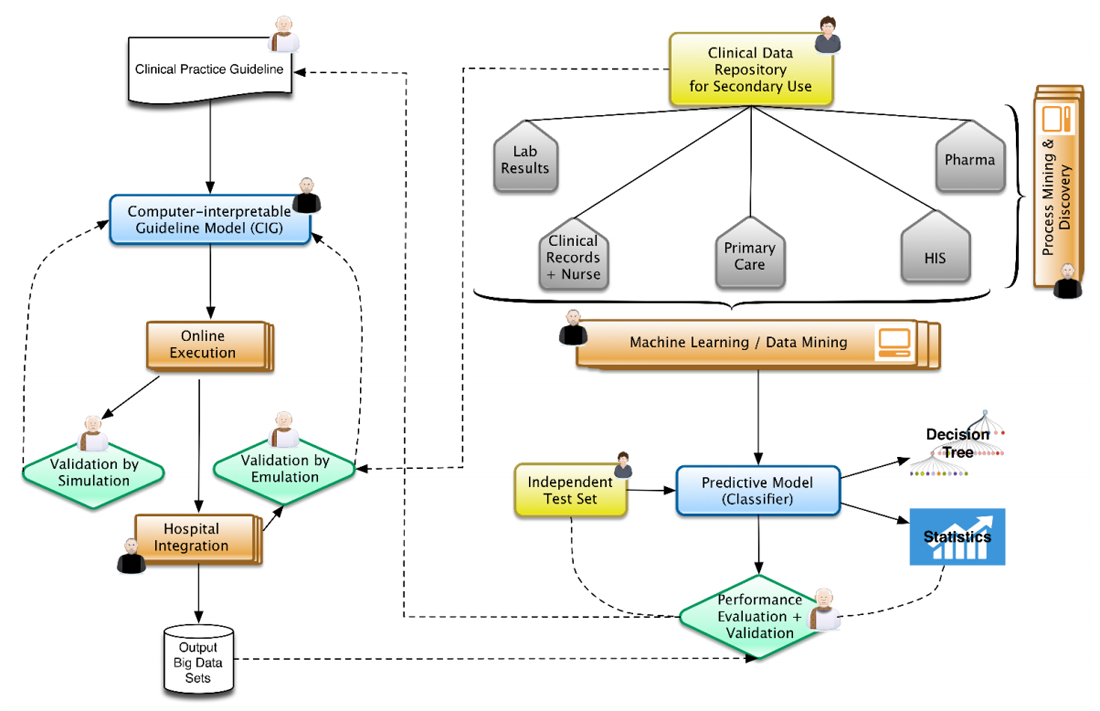
In this context, Figure 1 represents the architecture that represents our view, well aligned to positions already expressed by some research communities.[13] From top to bottom and left to right, physicians and epidemiologists develop CPGs that can be computerized, together with knowledge engineers, into computer-interpretable guideline (CIG) models. With the proper validation mechanisms, using data previously aggregated into clinical data repositories, these models can be trialed, after the corresponding integration into hospital information systems. The execution of CIG models can start generating data sets that are composed of acceptance or denials by physicians of recommendations (e.g., diagnosis, drug prescriptions, therapies, etc.) provided by the knowledge-based DSS developed, and treatments paths followed for different patient profiles. These paths can later on be analyzed by means of process mining techniques[14][15], unveiling common practices followed while using decision support and comparing the compliance of traditional clinical practice with the one recommended by the evidence-based DSS. At the same time, normalized clinical data repositories, while ensuring the quality of the data stored, can be used in the traditional view of machine learning and big data research.[16][17] The results could be complemented by comparing them with the output data sets of the KB-DSS. The output of the research could provide new evidence to be included in new versions of the CPGs (continuous improvement).
|
Innovative projects in HUCSC
The Innovation Unit of Hospital Universitario Clínico San Carlos, being transversal to the healthcare institution, is intended to cover two main aspects of innovation, always pursuing to increase value. On the one hand, it is expected to help hospital professionals to get their research into the mainstream, when there is an opportunity for it. On the other hand, it maintains a technical department to develop innovative products and test their prototypes, driving the hospital to maximize the possibilities that technological solutions could provide, especially artificial intelligence-based tools.
The ultimate intention is to disseminate the existence of these techniques while facilitating its understanding, create a culture of innovation within the hospital, and, when possible, get external companies to finalize these prototypes (or collaborate in the development) if they are demonstrated relevant and close to market possibility. The following are several ongoing projects aligned with these goals and that contribute several methods and artifacts to the architecture presented.
Computer-interpretable guideline for diagnosis and treatment of hyponatremia
The endocrinology department demanded a process-based solution to help new residents to improve their ability to diagnose and manage the hyponatremia condition (presenting low levels of serum sodium). Hyponatremia is the most frequent electrolyte disorder; however, according to some studies, it has proved to be very difficult to comprehend by physicians in general.[18] To address this project, we developed a CIG model[19][20] using the PROForma set of tools[21][22], covering the diagnosis of hyponatremia, classifying it into thirteen different subtypes. During a retrospective validation of the system with the data from 65 patients, we compared the system’s output to the diagnosis consensus of two experts, obtaining a very high agreement (kappa=0.86). The agreement found was also higher than a previous experiment found in the literature[23], carried out by comparing the performance of a resident physician — using the original paper guideline — with the diagnosis of senior physicians. Nonetheless, the most relevant advance of using such a system, beyond its successful diagnosis performance, was the identification and recording of data cases that were contrary to the consensus of international hyponatremia experts, specifically regarding hypoaldosteronism, where concrete marker thresholds were thought to be associated to its diagnosis. The application of our model found several cases where this hypothesis did not apply, showing the lack of real evidence and the need for further research. This is a concrete demonstration of how putting into practice these knowledge-based systems can help detect where evidence is failing and focus new research directions.
References
- ↑ Institute of Medicine (1990). Field, M.J.; Lohr, K.N.. ed. Clinical Practice Guidelines: Directions for a New Program. National Academies Press. doi:10.17226/1626.
- ↑ 2.0 2.1 Moskowitz, A.; McSparron, J.; Stone, D.J.; Celi, L.A. (2015). "Preparing a New Generation of Clinicians for the Era of Big Data". Harvard Medical Student Review 2 (1): 24–27. PMC PMC4327872. PMID 25688383. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4327872.
- ↑ Toussi, M.; Lamy, J.B.; Le Toumelin, P.; Venot, A. (2009). "Using data mining techniques to explore physicians' therapeutic decisions when clinical guidelines do not provide recommendations: Methods and example for type 2 diabetes". BMC Medical Informatics and Decision Making 9: 28. doi:10.1186/1472-6947-9-28. PMC PMC2700100. PMID 19515252. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2700100.
- ↑ Gabbay, J.; le May, A. (2004). "Evidence based guidelines or collectively constructed "mindlines?": Ethnographic study of knowledge management in primary care". BMJ 329 (7473): 1013. doi:10.1136/bmj.329.7473.1013. PMC PMC524553. PMID 15514347. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC524553.
- ↑ Handler, T.; Hieb, B. (13 June 2007). "The Updated Gartner CPR Generation Criteria" (PDF). Gartner Teleconference. Gartner. https://hiriresearch.files.wordpress.com/2011/04/cpr-generational-model.pdf.
- ↑ Greenes, R.A. (2015). "Evolution and Revolution in Knowledge-Driven Health IT: A 50-Year Perspective and a Look Ahead". In Riaño, D.; Lenz, R.; Miksch, S. et al.. Knowledge Representation for Health Care. Lecture Notes in Computer Science. 9485. Springer. pp. 3–20. doi:10.1007/978-3-319-26585-8_1. ISBN 9783319265858.
- ↑ Wang, D.; Peleg, M.; Tu, S.W. et al. (2004). "Design and implementation of the GLIF3 guideline execution engine". Journal of Biomedical Informatics 37 (5): 305–18. doi:10.1016/j.jbi.2004.06.002. PMID 15488745.
- ↑ Peleg, M. (2013). "Computer-interpretable clinical guidelines: A methodological review". Journal of Biomedical Informatics 46 (4): 744–63. doi:10.1016/j.jbi.2013.06.009. PMID 23806274.
- ↑ González-Ferrer, A.; Peleg, M. (2015). "Understanding requirements of clinical data standards for developing interoperable knowledge-based DSS: A case study". Computer Standards & Interfaces 42: 125–36. doi:10.1016/j.csi.2015.06.002.
- ↑ 10.0 10.1 Parimbelli, E.; Sacchi, L.; Bellazzi, R. (2016). "Decision Support through Data Integration: Strategies to Meet the Big Data Challenge". European Journal for Biomedical Informatics 12 (1): en10–en14. https://www.ejbi.org/archive/ejbi-volume-12-issue-1-year-2016.html.
- ↑ Mandel, J.C.; Kreda, D.A.; Mandl, K.D. et al. (2016). "SMART on FHIR: A standards-based, interoperable apps platform for electronic health records". JAMIA 23 (5): 899-908. doi:10.1093/jamia/ocv189. PMC PMC4997036. PMID 26911829. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4997036.
- ↑ Peleg, M.; Shahar, Y.; Quaglini, S. (2013). "Making healthcare more accessible, better, faster, and cheaper: The MobiGuide Project". European Journal of ePractice (20): 5–20. https://joinup.ec.europa.eu/sites/default/files/document/2014-06/ePractice%20Journal-Vol.20-November%202013.pdf.
- ↑ Lenz, R.; Peleg, M.; Reichert, M. (2012). "Healthcare Process Support: Achievements, Challenges, Current Research". International Journal of Knowledge-Based Organizations 2 (4): i–xvi. https://www.igi-global.com/journal/international-journal-knowledge-based-organizations/1177.
- ↑ van der Aalst, W.; Adriansyah, A.; Alves de Medeiros, A.K. et al. (2012). "Process Mining Manifesto". In Daniel, F.; Barkaoui, K.; Dustdar, S.. Business Process Management Workshops 2011. Lecture Notes in Business Information Processing. 99. Springer. doi:10.1007/978-3-642-28108-2_19. ISBN 9783642281082.
- ↑ Mans, R.S.; van der Aalst, W.; Vanwersch, R.J.B. et al. (2013). "Process Mining in Healthcare: Data Challenges When Answering Frequently Posed Questions". In Lenz, R.; Miksch, S.; Peleg, M. et al.. Process Support and Knowledge Representation in Health Care. Lecture Notes in Computer Science. 7738. Springer. doi:10.1007/978-3-642-36438-9_10. ISBN 9783642364389.
- ↑ Bellazzi, R.; Zupan, B. (2008). "Predictive data mining in clinical medicine: Current issues and guidelines". International Journal of Medical Informatics 77 (2): 81–97. doi:10.1016/j.ijmedinf.2006.11.006. PMID 17188928.
- ↑ Bellazzi, R.; Ferrazzi, F.; Sacchi, L. (2011). "Predictive data mining in clinical medicine: A focus on selected methods and applications". Wiley Interdisciplinary Reviews: Data Mining and Knowledge Discovery 1 (5): 416–30. doi:10.1002/widm.23.
- ↑ Dawson-Saunders, B.; Feltovich, P.J.; Coulson, R.L.; Steward, D.E. (1990). "A survey of medical school teachers to identify basic biomedical concepts medical students should understand". Academic Medicine 65 (7): 448–54. PMID 2242199.
- ↑ González-Ferrer, A.; Valcárcel, M.; Cháfer, J. et al. (2016). "Diagnóstico y tratamiento de hiponatremia usando modelos computacionales de guías de práctica clínica". Actas del XIX Congreso Nacional de Informática para la Salud, INFORSALUD 2016: 193–198.
- ↑ González-Ferrer, A.; Valcárcel, M.; Cuesta, M. et al. (2017). "Development of a computer-interpretable clinical guideline model for decision support in the differential diagnosis of hyponatremia". International Journal of Medical Informatics 103: 55–64. doi:10.1016/j.ijmedinf.2017.04.014. PMID 28551002.
- ↑ Fox, J.; Johns, N.; Rahmanzadeh, A. et al. (1998). "Disseminating medical knowledge: The PROforma approach". Artificial Intelligence in Medicine 14 (1–2): 157–81. PMID 9779888.
- ↑ Fox, J.; Gutenstein, M.; Khan, O. et al. (2015). "OpenClinical.net: A platform for creating and sharing knowledge and promoting best practice in healthcare". Computers in Industry 66: 63–72. doi:10.1016/j.compind.2014.10.001.
- ↑ Fenske, W.; Maier, S.K.; Blechschmidt, A. et al. (2010). "Utility and limitations of the traditional diagnostic approach to hyponatremia: A diagnostic study". American Journal of Medicine 123 (7): 652–7. doi:10.1016/j.amjmed.2010.01.013. PMID 20609688.
Notes
This presentation is faithful to the original, with only a few minor changes to grammar, spelling, and presentation, including the addition of PMCID and DOI when they were missing from the original reference.