Difference between revisions of "Journal:Laboratory information management system for the biosafety laboratory: Safety and efficiency"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 41: | Line 41: | ||
This paper proposes a laboratory information management system for the modern biosafety laboratory (BSL-LIMS), specifically designed for both [[Biosafety level|high-level biosafety]] labs (BSL-3 or -4) and basic research BSLs. Since the BSL-LIMS has its root in the specific needs of BSLs, the focus of the system is different from the traditional LIMS. Biosafety and biosecurity are made the primary concerns in the BSL-LIMS, with similar if not more importance than improving efficiency.<ref>{{Cite journal |last=Trevan |first=Tim |date=2015-11 |title=Biological research: Rethink biosafety |url=http://www.nature.com/articles/527155a |journal=Nature |language=en |volume=527 |issue=7577 |pages=155–158 |doi=10.1038/527155a |issn=0028-0836}}</ref> | This paper proposes a laboratory information management system for the modern biosafety laboratory (BSL-LIMS), specifically designed for both [[Biosafety level|high-level biosafety]] labs (BSL-3 or -4) and basic research BSLs. Since the BSL-LIMS has its root in the specific needs of BSLs, the focus of the system is different from the traditional LIMS. Biosafety and biosecurity are made the primary concerns in the BSL-LIMS, with similar if not more importance than improving efficiency.<ref>{{Cite journal |last=Trevan |first=Tim |date=2015-11 |title=Biological research: Rethink biosafety |url=http://www.nature.com/articles/527155a |journal=Nature |language=en |volume=527 |issue=7577 |pages=155–158 |doi=10.1038/527155a |issn=0028-0836}}</ref> | ||
Biosafety in laboratories has two major considerations: the safety of those who work in the lab in some capacity and the safety of the overall environment.<ref>{{Cite book |date=2004 |editor-last=World Health Organization |title=Laboratory biosafety manual |url=https://www.worldcat.org/title/mediawiki/oclc/ocm57658996 |edition=3rd ed |publisher=World Health Organization |place=Geneva |isbn=978-92-4-154650-8 |oclc=ocm57658996}}</ref> As such, the aim of a BSL is to keep both the personnel and environment safe before, during, and after experiments and other analyses. If there are any unavoidable safety risks in the course of a research project, then the risks must be reduced to an acceptable and controllable degree before the start of the project, and be kept this way until the end of it; otherwise, the project should be abandoned rather than allowed to begin. Hence, as a part of BSL activities, the design of the BSL-LIMS has to comply with the safety standards set by BSLs. Since biosafety is a precondition for experiments in BSLs, the focus of the corresponding LIMS shifts from post-experimental information (i.e., results) management to pre-experimental information (i.e., preparation) management. Even though the overall framework of the BSL-LIMS might look similar to that of a traditional LIMS, their contents would be very different. | Biosafety in laboratories has two major considerations: the safety of those who work in the lab in some capacity and the safety of the overall environment.<ref name=":6">{{Cite book |date=2004 |editor-last=World Health Organization |title=Laboratory biosafety manual |url=https://www.worldcat.org/title/mediawiki/oclc/ocm57658996 |edition=3rd ed |publisher=World Health Organization |place=Geneva |isbn=978-92-4-154650-8 |oclc=ocm57658996}}</ref> As such, the aim of a BSL is to keep both the personnel and environment safe before, during, and after experiments and other analyses. If there are any unavoidable safety risks in the course of a research project, then the risks must be reduced to an acceptable and controllable degree before the start of the project, and be kept this way until the end of it; otherwise, the project should be abandoned rather than allowed to begin. Hence, as a part of BSL activities, the design of the BSL-LIMS has to comply with the safety standards set by BSLs. Since biosafety is a precondition for experiments in BSLs, the focus of the corresponding LIMS shifts from post-experimental information (i.e., results) management to pre-experimental information (i.e., preparation) management. Even though the overall framework of the BSL-LIMS might look similar to that of a traditional LIMS, their contents would be very different. | ||
Information management in a BSL can be divided into four categories: | Information management in a BSL can be divided into four categories: | ||
| Line 88: | Line 88: | ||
|} | |} | ||
|} | |} | ||
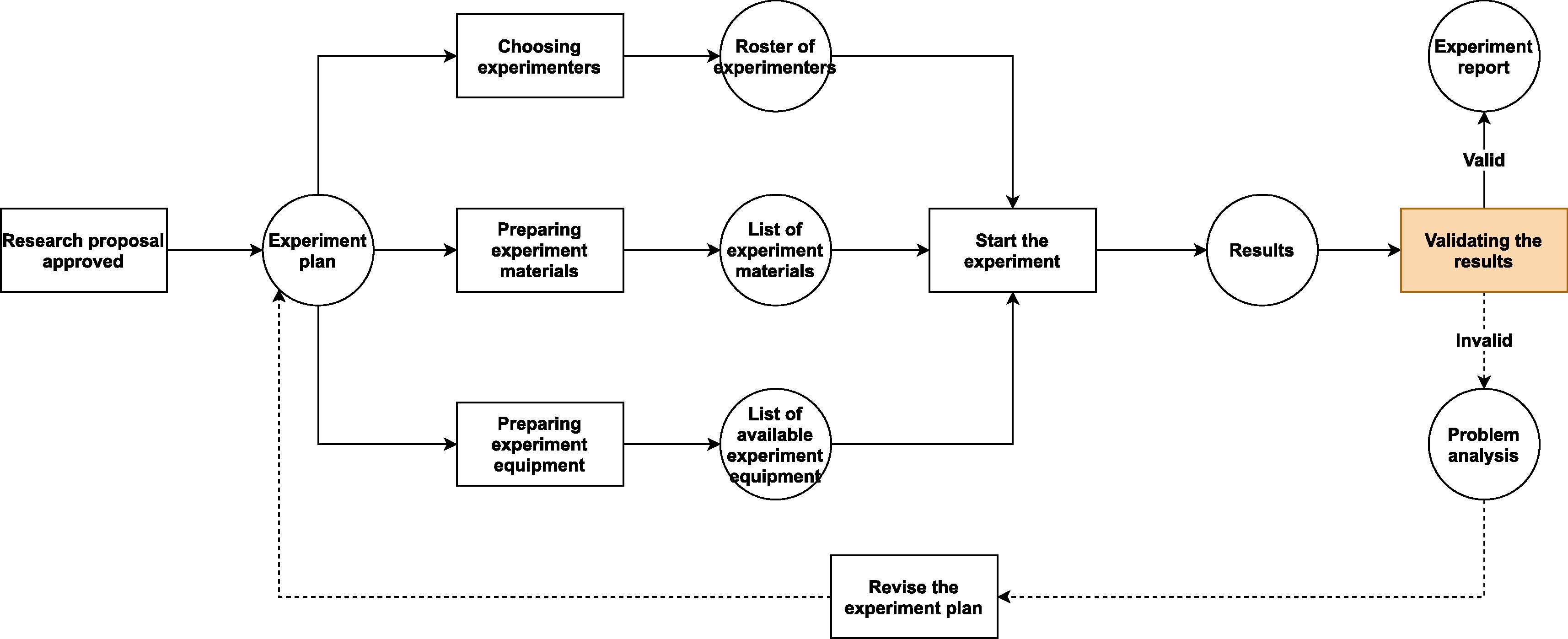
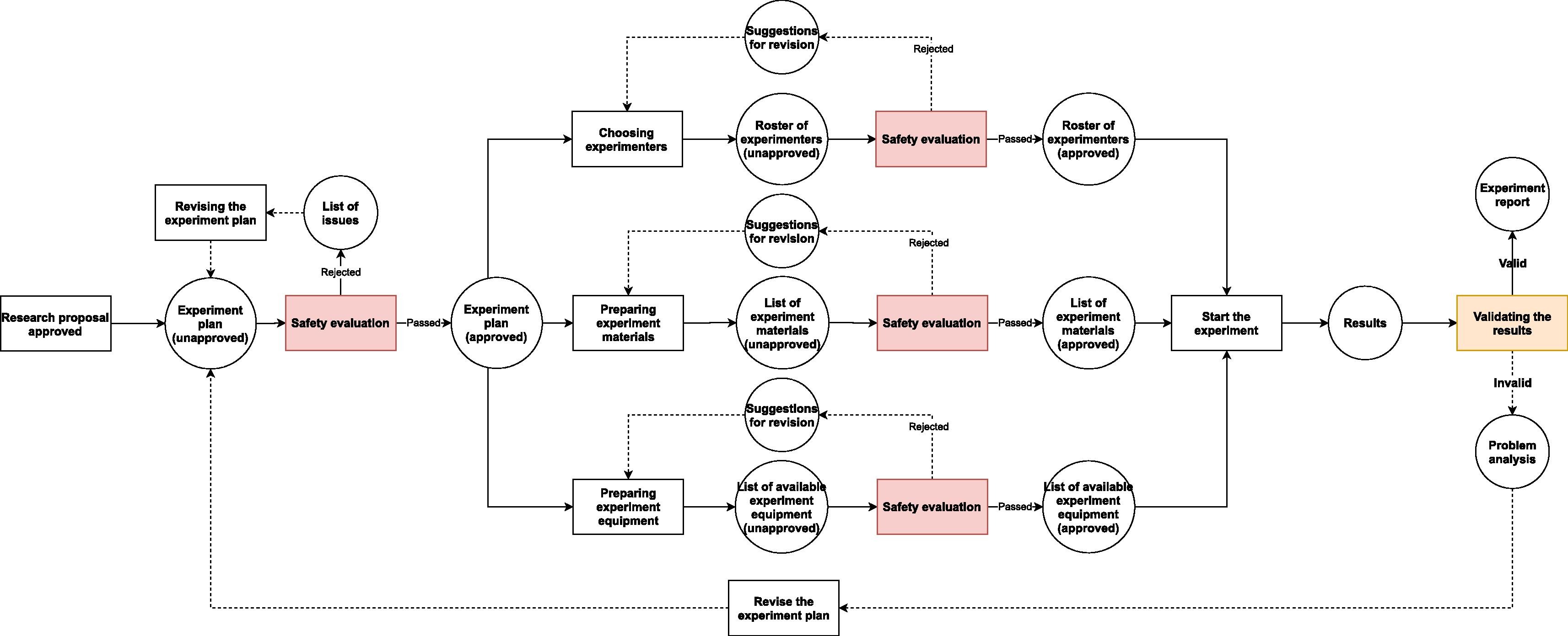
Because the workflows in a research BSL (Fig. 2.) are similar to the ones in a regular multi-purpose biological laboratory (Fig. 1), only with some additional safety checkpoints, the BSL-LIMS can refer to some well-recognized standards (e.g., the [[International Organization for Standardization]] [ISO] and Clinical & Labs Standards Institute [CLSI]) and/or previous studies<ref name=":0" /><ref name=":1" /><ref name=":2" /><ref name=":3" /><ref>{{Cite web |last=ASTM International |date=2018 |title=ASTM E1578 - 18 Standard Guide for Laboratory Informatics |url=https://www.astm.org/Standards/E1578.htm |publisher=ASTM International}}</ref> to design the modules that are in common to all biological labs (e.g., project-template design, experiment scheduling, sample management, experimental data collection, results analysis and reporting, etc.). In contrast, for the processes that are unique to the BSL, there is no previous standard or study that can be used as examples. Thus, we have to create new modules or update existing LIMS modules to satisfy the requirements of safety information management, based on institutional, national, or international biosafety guidelines.<ref name=":6" /><ref name="GB 19489-2008">{{cite web |url=https://www.codeofchina.com/standard/GB19489-2008.html |title=GB 19489-2008 Laboratories - General Requirements for Biosafety (English Version) |author=AQSIQ |work=Code of China |date=26 December 2008}}</ref><ref name="RegMicrobe16">{{cite web |url=http://www.lawinfochina.com/display.aspx?lib=law&id=3849&CGid= |title=Regulation on the Bio-safety Management of Pathogenic Microbe Labs - Revised |author=State Council of China |work=lawinfochina.com |date=06 February 2016}}</ref> | |||
Project management can be divided into two parts, based on its effective stage. | |||
====The safety modules for project preparation==== | |||
==References== | ==References== | ||
Revision as of 21:03, 22 October 2021
| Full article title | Laboratory information management system for the biosafety laboratory: Safety and efficiency |
|---|---|
| Journal | Journal of Biosafety and Biosecurity |
| Author(s) | Sun, Dingzhong; Wu, Linhuan; Fan, Guomei |
| Author affiliation(s) | Institute of Microbiology Chinese Academy of Sciences |
| Primary contact | Email: wulh at im dot ac dot cn |
| Year published | 2021 |
| Volume and issue | 3(1) |
| Page(s) | 28–34 |
| DOI | 10.1016/j.jobb.2021.03.001 |
| ISSN | 2588-9338 |
| Distribution license | Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International |
| Website | https://www.sciencedirect.com/science/article/pii/S2588933821000042 |
| Download | https://www.sciencedirect.com/science/article/pii/S2588933821000042/pdfft (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
The laboratory information management system (LIMS) has been widely used to facilitate laboratory activities. However, the current LIMS does not contain functions to improve the safety of laboratory work, which is a major concern of biosafety laboratories (BSLs). With significant biosafety information needing to be managed and an increasing number of biosafety-related research projects underway, it is worthy of expanding the current framework of LIMS and building a system that is more suitable for BSL usage. Such a system should carefully trade off between the safety and efficiency of regular lab activities, allowing laboratory staff to conduct their research as freely as possible while also ensuring their and the environment’s safety. In order to achieve this goal, relevant information on the type of research, laboratory personnel, experimental materials, and experimental equipment must be collected and fully utilized by a centralized system and its databases.
Keywords: laboratory information management system, biosafety, biological research laboratory, laboratory safety, workflow management
Introduction
A laboratory information management system (LIMS) is a computerized system that collects, processes, and stores laboratory-generated data and information. Though the LIMS was initially developed to automate the management of experimental data, it now has the potential to develop into the digital hub of many laboratory activities.[1][2][3][4] Inside the lab, by deeply entwining the LIMS and highly-automated laboratory equipment, researchers are even able to program robots (for example, the Biomek series from Beckman Coulter) to execute iterative experiments without any human operation.[5] Outside the lab, many current LIMS in clinical and analytical labs have greatly accelerated the process of releasing test results to the customer because of their network-based reporting system.[4] Regardless of the specific functions a LIMS possesses, the ultimate purpose of the LIMS is to save human labor and improve data quality (i.e., accuracy, reliability, and timeliness).[6]
Although the importance of a LIMS in a lab was quite low initially, with the rapid development of computer and network technologies, the LIMS had gradually evolved to play more diverse roles and become an irreplaceable part in many labs. The range of LIMS users has expanded as well, from mostly analytical labs to diagnostic biosafety laboratories (BSLs) of specific function, as well as more general research labs.[2][3][7][8]
Nevertheless, the functions of all current LIMS, to our knowledge, do not address the major concern of biological safety and security in biosafety labs. This is even true for those LIMS currently used in BSLs. Although the modern LIMS has reduced a number of human factors from certain experimental activities, few if any have actually increased the general safety of the BSL as these systems do not contain any safety- and security-control mechanisms. The focus of a traditional LIMS, whether it is used in a BSL or not, is still to better facilitate lab staff performing experiments. Even the electronic laboratory notebook (ELN), a tool widely appreciated by BSL staff, was not designed for but merely happened to suit the working environment of BSLs. As such, we do not currently have any digital systems that can help a BSL manage its biosafety information. At the same time, biosafety-related information has been collected, stored, and transmitted in digital form in BSLs since at least 2000, and the amount of digital biosafety information is growing, when compared to paper-based information.[9] This has resulted in a strange situation, onw where biosafety information itself is highly digitalized, yet there is no centralized system designed to better organize that electronic information and enable laboratorians to use it appropriately. Most information typically stays in the form of isolated electronic documents on either a local computer or hosted on a local network, and the search and retrieval of the information is usually complicated. For example, when a safety document is requested by a user, the search, deployment, use, and dissemination of the document is largely done on an individual basis, via a not-well-indexed information system and an unrelated transmission system like email or even paper-based messaging. Hence, the information management system of biosafety labs—if the system even exists—is ineffective, inefficient, and cannot satisfy the needs of the modern biosafety lab. We therefore believe that a specific information management system for biosafety laboratories that is able to improve the safety and efficiency of these laboratories is a necessity.
A laboratory information management system for the biosafety laboratory
This paper proposes a laboratory information management system for the modern biosafety laboratory (BSL-LIMS), specifically designed for both high-level biosafety labs (BSL-3 or -4) and basic research BSLs. Since the BSL-LIMS has its root in the specific needs of BSLs, the focus of the system is different from the traditional LIMS. Biosafety and biosecurity are made the primary concerns in the BSL-LIMS, with similar if not more importance than improving efficiency.[10]
Biosafety in laboratories has two major considerations: the safety of those who work in the lab in some capacity and the safety of the overall environment.[11] As such, the aim of a BSL is to keep both the personnel and environment safe before, during, and after experiments and other analyses. If there are any unavoidable safety risks in the course of a research project, then the risks must be reduced to an acceptable and controllable degree before the start of the project, and be kept this way until the end of it; otherwise, the project should be abandoned rather than allowed to begin. Hence, as a part of BSL activities, the design of the BSL-LIMS has to comply with the safety standards set by BSLs. Since biosafety is a precondition for experiments in BSLs, the focus of the corresponding LIMS shifts from post-experimental information (i.e., results) management to pre-experimental information (i.e., preparation) management. Even though the overall framework of the BSL-LIMS might look similar to that of a traditional LIMS, their contents would be very different.
Information management in a BSL can be divided into four categories:
- Project management
- Personnel administration
- Experimental material management
- Equipment management
As such, any information management system would involve a large amount of information collection and exchange within and between different categories. Many checkpoints would need to be set up for the sake of safety control. These interconnected information flows and checkpoints would then form an elaborate network whose structure can be simplified to Petri Nets[2], with laboratory data or information as places and research or audit activities as transitions. Since laboratory workflow management has already been a part of many regular research labs’ information management systems[2][3][7], some modules in these traditional LIMS (e.g., the management of samples, materials, or equipment) may be used by the BSL-LIMS, though new databases on biosafety information should be added, and existing structures probably need to be redesigned in order to meet the new demands for improving biosafety and biosecurity.
Project management
Research projects comprise the bulk of a research laboratory's activities. In other words, a research lab is founded so as to support a succession of research project. Hence, the effective management of projects is the most important job for a lab manager or director. However, there are two new challenges to meet to get the job done in high-level BSLs, in addition to the challenges raised in regular laboratories.
First, high-level BSLs are usually multi-purpose research laboratories that conduct diverse studies. Unlike diagnostic or analytical laboratories, whose type of work is usually consistently the same, the projects within a research lab usually change in their nature. In a diagnostic or analytical laboratory, a LIMS can be adopted to manage lab activities once the diagnostic or analytical processes have been determined.[4] Since the information flows in these laboratories usually follow a linear structure, once the LIMS has been installed and connected with automated devices, it can then take its role as the information hub and quality control center of the laboratory.[6] In contrast, BSLs or general research labs comparatively change their experimental activities often, and therefore no single procedure is enough to keep laboratory activities safe and successful. As a result, the LIMS would get downplayed to an ELN or a data reporter attached to a certain piece of automated equipment,[2][8] losing its central position as the information hub of the lab, that is unless we can expand the LIMS' functionality.[7]
Fortunately, adding the new functions required to make a LIMS adapt to BSL usage is not difficult. All we need to do is to step back a bit and take a look at the bigger picture. From a principal investigator’s point of view, all research activities follow certain pattern (Fig. 1.). If we can expand the system's audit trail and revision control—typically core functions in the LIMS—from managing only "scientific data" to "all data that could affect scientific research," then we should be able to create a system that is able to oversee all research projects in a laboratory. Each of these projects contains three aspects to be managed: the experimenters, the experiment materials, and the experiment equipment and facilities. All of these aspects are affected by the details of the specific project, and the quality of preparation for these aspects will predetermine whether the project will go well or not.
|
Secondly, in regular biological labs, the lab director or other person in charge may do an inspection of the experimenters, the experiment materials, and the experiment equipment and facilities at the beginning of the project. However, this kind of inspection is mostly voluntary and rigid, and the inspected contents are usually arbitrary. There is no mechanism to secure compliance with certain operational rules before and during the project, since it lacks nationally or institutionally enforced laws or regulations. As a result, few lab workers and principal investigators would attempt to make sure that everything that would be involved in the project is well prepared before they start the first experiment; instead, more would try to get started first as soon as the basic conditions of the project has been met, and the complete fulfilment of the experimental conditions would be considered to be a parallel task. This mode might work in some of the regular research labs, but it should be avoided by every BSL.
In regular biological labs, since contagious materials or other potentially dangerous self-propagating materials (e.g., gene drive materials) are excluded from experiments, the causes of accidents are usually due to non-biological factors. These factors may lead to very serious consequences, but the range of their influence is mostly limited to the lab itself and its workers. For instance, centrifugation is one of the common activities that can lead to severe injuries or even death of people.[12] Nonetheless, the damage caused by centrifuge failure can hardly go beyond the neighboring rooms where the centrifuge is located and usually only has short-term detrimental effect. In contrast, the leakage of untreated contagious materials from BSLs can lead to massive infection far beyond laboratory workers and last as long as the chain of transmission has not been broken. Even a leakage from a teaching lab of low biosafety level may cause infection in a large community.[13] Hence, BSLs need extra safety measures to counter the increased risk and lower the rate of accidents—especially infection-related accidents—to the minimum (Fig. 2.).
However, there are several potential downsides for doing so. First, adding extra authorization and inspection processes will cost scientists extra time. The increase in the time that they have to spend on non-research affairs will lower their efficiency and subsequently reduce the project's output. Second, humans are prone to errors and thereby can make more mistakes when they are more burdened. Although the aim of extra safety regulations and rules is to lower risk, the increased amount of precautions also adds to the burden of both research staff and biosafety managing staff, making them prone to becoming exhausted by the extra safety information that they need to deal with. This reduction in mental and physical faculties is often a cause of laboratory accidents.[14] Therefore, we need a tireless worker to support human activities, lowering the work load and errors of humans. And that is exactly what a BSL-LIMS can offer.
|
Because the workflows in a research BSL (Fig. 2.) are similar to the ones in a regular multi-purpose biological laboratory (Fig. 1), only with some additional safety checkpoints, the BSL-LIMS can refer to some well-recognized standards (e.g., the International Organization for Standardization [ISO] and Clinical & Labs Standards Institute [CLSI]) and/or previous studies[2][3][4][7][15] to design the modules that are in common to all biological labs (e.g., project-template design, experiment scheduling, sample management, experimental data collection, results analysis and reporting, etc.). In contrast, for the processes that are unique to the BSL, there is no previous standard or study that can be used as examples. Thus, we have to create new modules or update existing LIMS modules to satisfy the requirements of safety information management, based on institutional, national, or international biosafety guidelines.[11][16][17]
Project management can be divided into two parts, based on its effective stage.
The safety modules for project preparation
References
- ↑ Gibbon, Gerst A. (1 May 1996). "A brief history of LIMS" (in en). Laboratory Automation & Information Management 32 (1): 1–5. doi:10.1016/1381-141X(95)00024-K. https://linkinghub.elsevier.com/retrieve/pii/1381141X9500024K.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Goodman, N.; Rozen, S.; Stein, L. D. (1998). "The LabFlow system for workflow management in large scale biology research laboratories". Proceedings. International Conference on Intelligent Systems for Molecular Biology 6: 69–77. ISSN 1553-0833. PMID 9783211. https://pubmed.ncbi.nlm.nih.gov/9783211.
- ↑ 3.0 3.1 3.2 3.3 Ludäscher, Bertram; Altintas, Ilkay; Berkley, Chad; Higgins, Dan; Jaeger, Efrat; Jones, Matthew; Lee, Edward A.; Tao, Jing et al. (25 August 2006). "Scientific workflow management and the Kepler system" (in en). Concurrency and Computation: Practice and Experience 18 (10): 1039–1065. doi:10.1002/cpe.994. ISSN 1532-0626. https://onlinelibrary.wiley.com/doi/10.1002/cpe.994.
- ↑ 4.0 4.1 4.2 4.3 Skobelev, D. O.; Zaytseva, T. M.; Kozlov, A. D.; Perepelitsa, V. L.; Makarova, A. S. (1 January 2011). "Laboratory information management systems in the work of the analytic laboratory" (in en). Measurement Techniques 53 (10): 1182–1189. doi:10.1007/s11018-011-9638-7. ISSN 0543-1972. http://link.springer.com/10.1007/s11018-011-9638-7.
- ↑ McDonald, Michael J.; Rice, Daniel P.; Desai, Michael M. (1 March 2016). "Sex speeds adaptation by altering the dynamics of molecular evolution" (in en). Nature 531 (7593): 233–236. doi:10.1038/nature17143. ISSN 0028-0836. PMC PMC4855304. PMID 26909573. http://www.nature.com/articles/nature17143.
- ↑ 6.0 6.1 Organization, World Health; World Health Organization, Centers for Disease Control and Prevention (U.S.); World Health Organization; Clinical and Laboratory Standards Institute (2011). Laboratory Quality Management System. Geneva: World Health Organization. ISBN 978-92-4-068857-5. OCLC 1162457940. https://public.ebookcentral.proquest.com/choice/publicfullrecord.aspx?p=1142071.
- ↑ 7.0 7.1 7.2 7.3 Melo, Alexandre; Faria-Campos, Alessandra; DeLaat, Daiane; Keller, Rodrigo; Abreu, Vinícius; Campos, Sérgio (2010). "SIGLa: an adaptable LIMS for multiple laboratories" (in en). BMC Genomics 11 (Suppl 5): S8. doi:10.1186/1471-2164-11-S5-S8. ISSN 1471-2164. PMC PMC3045801. PMID 21210974. http://bmcgenomics.biomedcentral.com/articles/10.1186/1471-2164-11-S5-S8.
- ↑ 8.0 8.1 IGI Testing Consortium; Amen, Alexandra M.; Barry, Kerrie W.; Boyle, John M.; Brook, Cara E.; Choo, Seunga; Cornmesser, L. T.; Dilworth, David J. et al. (1 July 2020). "Blueprint for a pop-up SARS-CoV-2 testing lab" (in en). Nature Biotechnology 38 (7): 791–797. doi:10.1038/s41587-020-0583-3. ISSN 1087-0156. http://www.nature.com/articles/s41587-020-0583-3.
- ↑ Stuart, Ralph; Stewart, James; Herrick, Robert (29 March 2019), Mansdorf, S. Z., ed., "INFORMATION RESOURCES FOR OCCUPATIONAL SAFETY AND HEALTH PROFESSIONALS" (in en), Handbook of Occupational Safety and Health (Hoboken, NJ, USA: John Wiley & Sons, Inc.): 37–48, doi:10.1002/9781119581482.ch2, ISBN 978-1-119-58148-2, https://onlinelibrary.wiley.com/doi/10.1002/9781119581482.ch2. Retrieved 2021-10-22
- ↑ Trevan, Tim (1 November 2015). "Biological research: Rethink biosafety" (in en). Nature 527 (7577): 155–158. doi:10.1038/527155a. ISSN 0028-0836. http://www.nature.com/articles/527155a.
- ↑ 11.0 11.1 World Health Organization, ed. (2004). Laboratory biosafety manual (3rd ed ed.). Geneva: World Health Organization. ISBN 978-92-4-154650-8. OCLC ocm57658996. https://www.worldcat.org/title/mediawiki/oclc/ocm57658996.
- ↑ Kolff, J.; Ankney, R. N.; Wurzel, D.; Devineni, R. (1 September 1996). "Centrifugal pump failures". The Journal of Extra-Corporeal Technology 28 (3): 118–122. ISSN 0022-1058. PMID 10163498. https://pubmed.ncbi.nlm.nih.gov/10163498.
- ↑ Emmert, Elizabeth A. B.; the ASM Task Committee on Laboratory Biosafety (1 January 2013). "Biosafety Guidelines for Handling Microorganisms in the Teaching Laboratory: Development and Rationale" (in en). Journal of Microbiology & Biology Education 14 (1): 78–83. doi:10.1128/jmbe.v14i1.531. ISSN 1935-7877. PMC PMC3706168. PMID 23858356. https://journals.asm.org/doi/10.1128/jmbe.v14i1.531.
- ↑ Wurtz, N.; Papa, A.; Hukic, M.; Di Caro, A.; Leparc-Goffart, I.; Leroy, E.; Landini, M. P.; Sekeyova, Z. et al. (1 August 2016). "Survey of laboratory-acquired infections around the world in biosafety level 3 and 4 laboratories" (in en). European Journal of Clinical Microbiology & Infectious Diseases 35 (8): 1247–1258. doi:10.1007/s10096-016-2657-1. ISSN 0934-9723. PMC PMC7088173. PMID 27234593. http://link.springer.com/10.1007/s10096-016-2657-1.
- ↑ ASTM International (2018). "ASTM E1578 - 18 Standard Guide for Laboratory Informatics". ASTM International. https://www.astm.org/Standards/E1578.htm.
- ↑ AQSIQ (26 December 2008). "GB 19489-2008 Laboratories - General Requirements for Biosafety (English Version)". Code of China. https://www.codeofchina.com/standard/GB19489-2008.html.
- ↑ State Council of China (6 February 2016). "Regulation on the Bio-safety Management of Pathogenic Microbe Labs - Revised". lawinfochina.com. http://www.lawinfochina.com/display.aspx?lib=law&id=3849&CGid=.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. Nothing else was changed in accordance with the NoDerivatives portion of the license.