Difference between revisions of "Main Page/Featured article of the week/2021"
Shawndouglas (talk | contribs) (Added last week's article of the week) |
Shawndouglas (talk | contribs) (Added last week's article of the week) |
||
| Line 17: | Line 17: | ||
<!-- Below this line begin pasting previous news --> | <!-- Below this line begin pasting previous news --> | ||
<h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: July 5–11:</h2> | <h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: July 12–18:</h2> | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Tab1 Konnick PractLabMed2020 21.png|240px]]</div> | |||
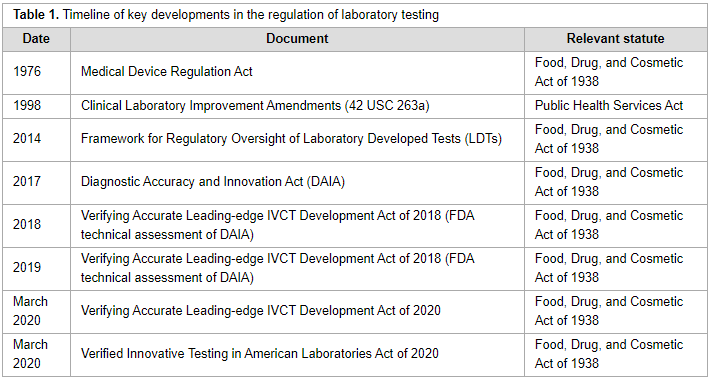
'''"[[Journal:The regulatory landscape of precision oncology laboratory medicine in the United States: Perspective on the past five years and considerations for future regulation|The regulatory landscape of precision oncology laboratory medicine in the United States: Perspective on the past five years and considerations for future regulation]]"''' | |||
The [[Regulatory compliance|regulatory]] landscape for precision [[oncology]] in the United States is complicated, with multiple governmental regulatory agencies with different scopes of jurisdiction. Several regulatory proposals have been introduced since the [[Food and Drug Administration]] released draft guidance to regulate [[laboratory developed test]]s in 2014. Key aspects of the most recent proposals and discussion of central arguments related to the regulation of precision oncology laboratory tests provides insight to stakeholders for future discussions related to regulation of [[laboratory]] [[Medical test|tests]]. ('''[[Journal:The regulatory landscape of precision oncology laboratory medicine in the United States: Perspective on the past five years and considerations for future regulation|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: July 5–11:</h2> | |||
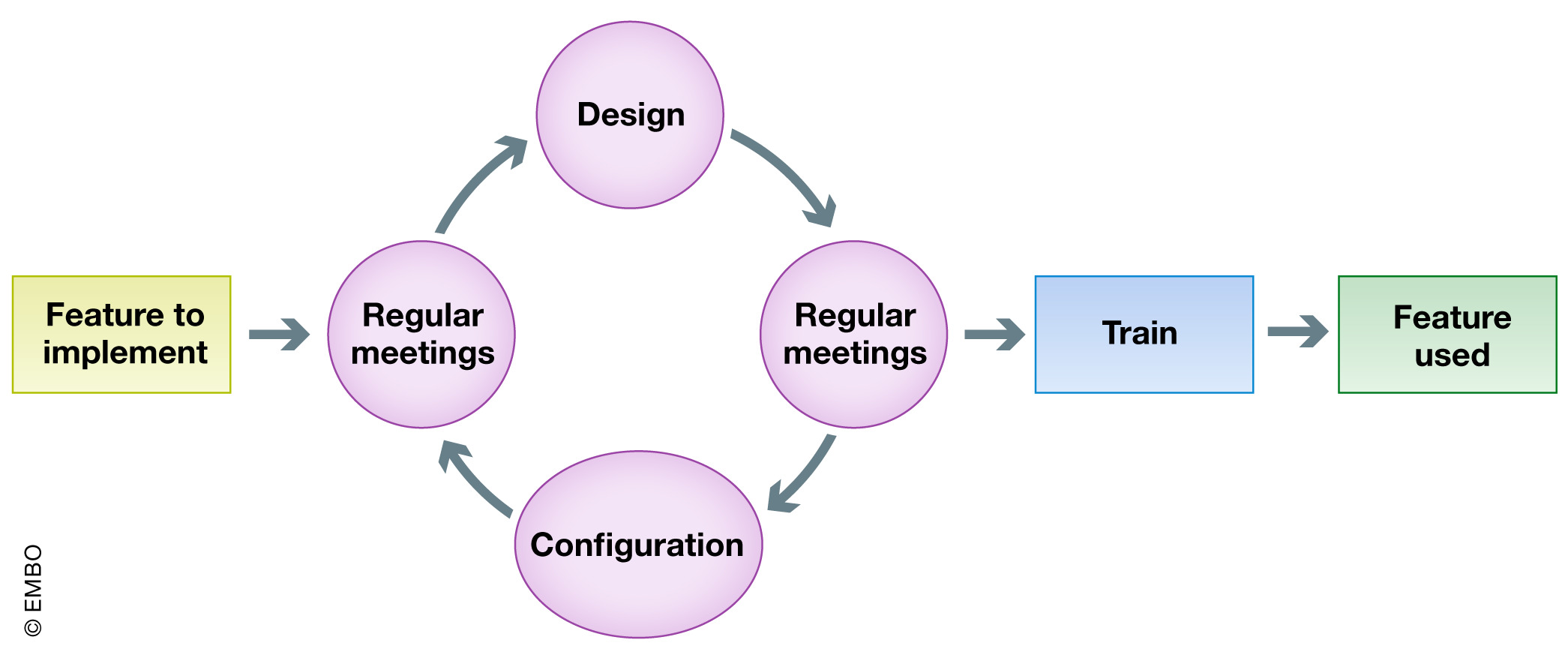
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig6 Argento EMBOReports2020 21-3.jpg|240px]]</div> | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig6 Argento EMBOReports2020 21-3.jpg|240px]]</div> | ||
'''"[[Journal:Institutional ELN-LIMS deployment: Highly customizable ELN-LIMS platform as a cornerstone of digital transformation for life sciences research institutes|Institutional ELN-LIMS deployment: Highly customizable ELN-LIMS platform as a cornerstone of digital transformation for life sciences research institutes]]"''' | '''"[[Journal:Institutional ELN-LIMS deployment: Highly customizable ELN-LIMS platform as a cornerstone of digital transformation for life sciences research institutes|Institutional ELN-LIMS deployment: Highly customizable ELN-LIMS platform as a cornerstone of digital transformation for life sciences research institutes]]"''' | ||
Revision as of 17:53, 19 July 2021
|
|
If you're looking for other "Article of the Week" archives: 2014 - 2015 - 2016 - 2017 - 2018 - 2019 - 2020 - 2021 |
Featured article of the week archive - 2021
Welcome to the LIMSwiki 2021 archive for the Featured Article of the Week.
Featured article of the week: July 12–18:The regulatory landscape for precision oncology in the United States is complicated, with multiple governmental regulatory agencies with different scopes of jurisdiction. Several regulatory proposals have been introduced since the Food and Drug Administration released draft guidance to regulate laboratory developed tests in 2014. Key aspects of the most recent proposals and discussion of central arguments related to the regulation of precision oncology laboratory tests provides insight to stakeholders for future discussions related to regulation of laboratory tests. (Full article...)
|