Difference between revisions of "Template:Article of the week"
Shawndouglas (talk | contribs) (Updated article of the week text) |
Shawndouglas (talk | contribs) (Updated article of the week text) |
||
| Line 1: | Line 1: | ||
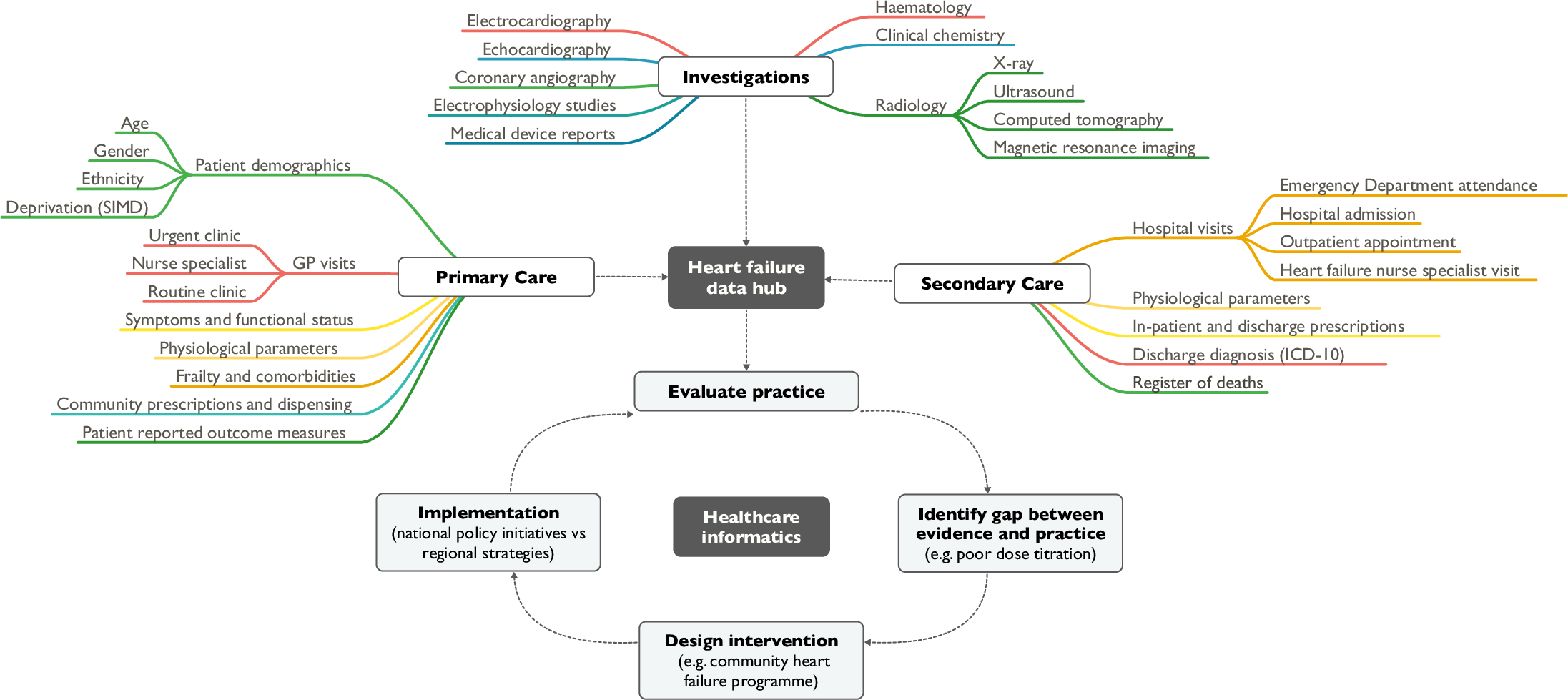
'''"[[Journal: | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Anwar PLOSMed2019 16-5.png|240px]]</div> | ||
'''"[[Journal:Heart failure and healthcare informatics|Heart failure and healthcare informatics]]"''' | |||
As biomedical research expands our armory of effective, evidence-based therapies, there is a corresponding need for high-quality implementation science—the study of strategies to integrate and embed research advances into [[Health care|clinical practice]]. Large-scale collection and analysis of routinely collected healthcare data may facilitate this in three main ways. Firstly, evaluation of key healthcare metrics can help to identify the areas of practice that differ most from guideline recommendations. Secondly, with sufficiently granular data, it may be possible to detect the underlying drivers of deficiencies in practice. Thirdly, longitudinal data collection should enable us to evaluate large-scale policy initiatives and compare the effectiveness of differing strategies on process and patient outcomes. ('''[[Journal:Heart failure and healthcare informatics|Full article...]]''')<br /> | |||
<br /> | <br /> | ||
''Recently featured'': | ''Recently featured'': | ||
: ▪ [[Journal:Cyberbiosecurity for biopharmaceutical products|Cyberbiosecurity for biopharmaceutical products]] | |||
: ▪ [[Journal:A bibliometric analysis of Cannabis publications: Six decades of research and a gap on studies with the plant|A bibliometric analysis of Cannabis publications: Six decades of research and a gap on studies with the plant]] | : ▪ [[Journal:A bibliometric analysis of Cannabis publications: Six decades of research and a gap on studies with the plant|A bibliometric analysis of Cannabis publications: Six decades of research and a gap on studies with the plant]] | ||
: ▪ [[Journal:Leaner and greener analysis of cannabinoids|Leaner and greener analysis of cannabinoids]] | : ▪ [[Journal:Leaner and greener analysis of cannabinoids|Leaner and greener analysis of cannabinoids]] | ||
Revision as of 14:56, 26 August 2019
"Heart failure and healthcare informatics"
As biomedical research expands our armory of effective, evidence-based therapies, there is a corresponding need for high-quality implementation science—the study of strategies to integrate and embed research advances into clinical practice. Large-scale collection and analysis of routinely collected healthcare data may facilitate this in three main ways. Firstly, evaluation of key healthcare metrics can help to identify the areas of practice that differ most from guideline recommendations. Secondly, with sufficiently granular data, it may be possible to detect the underlying drivers of deficiencies in practice. Thirdly, longitudinal data collection should enable us to evaluate large-scale policy initiatives and compare the effectiveness of differing strategies on process and patient outcomes. (Full article...)
Recently featured: