Difference between revisions of "Template:Article of the week"
Shawndouglas (talk | contribs) (Updated article of the week text.) |
Shawndouglas (talk | contribs) (Updated article of the week text.) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Sinard JPathologyInformatics2012 3.jpg|240px]]</div> | ||
'''"[[Journal: | '''"[[Journal:Custom software development for use in a clinical laboratory|Custom software development for use in a clinical laboratory]]"''' | ||
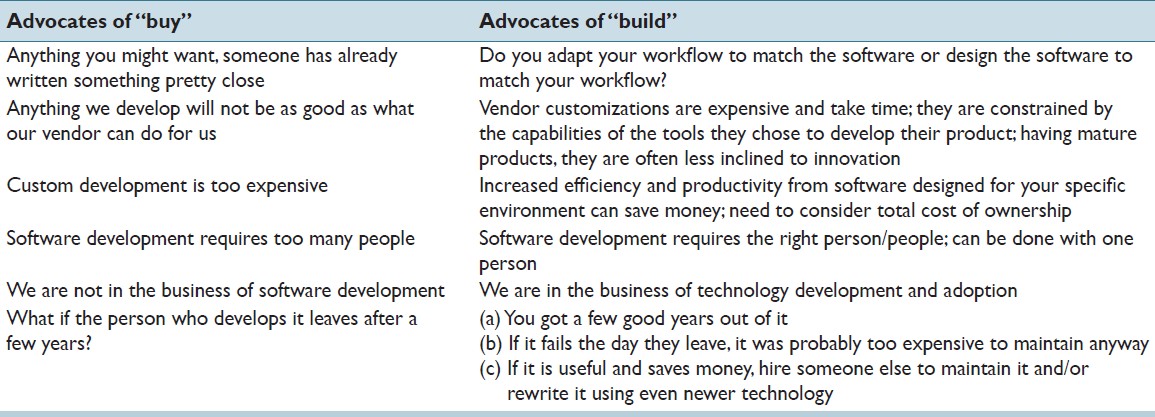
In-house software development for use in a [[clinical laboratory]] is a controversial issue. Many of the objections raised are based on outdated software development practices, an exaggeration of the risks involved, and an underestimation of the benefits that can be realized. Buy versus build analyses typically do not consider total costs of ownership, and unfortunately decisions are often made by people who are not directly affected by the workflow obstacles or benefits that result from those decisions. We have been developing custom software for clinical use for over a decade, and this article presents our perspective on this practice. A complete analysis of the decision to develop or purchase must ultimately examine how the end result will mesh with the departmental workflow, and custom-developed solutions typically can have the greater positive impact on efficiency and productivity, substantially altering the decision balance sheet. ('''[[Journal:Custom software development for use in a clinical laboratory|Full article...]]''')<br /> | |||
We | |||
<br /> | <br /> | ||
''Recently featured'': | ''Recently featured'': | ||
: ▪ [[Journal:NG6: Integrated next generation sequencing storage and processing environment|NG6: Integrated next generation sequencing storage and processing environment]] | |||
: ▪ [[Journal:STATegra EMS: An experiment management system for complex next-generation omics experiments|STATegra EMS: An experiment management system for complex next-generation omics experiments]] | : ▪ [[Journal:STATegra EMS: An experiment management system for complex next-generation omics experiments|STATegra EMS: An experiment management system for complex next-generation omics experiments]] | ||
: ▪ [[Journal:No specimen left behind: Industrial scale digitization of natural history collections|No specimen left behind: Industrial scale digitization of natural history collections]] | : ▪ [[Journal:No specimen left behind: Industrial scale digitization of natural history collections|No specimen left behind: Industrial scale digitization of natural history collections]] | ||
Revision as of 15:12, 16 May 2016
"Custom software development for use in a clinical laboratory"
In-house software development for use in a clinical laboratory is a controversial issue. Many of the objections raised are based on outdated software development practices, an exaggeration of the risks involved, and an underestimation of the benefits that can be realized. Buy versus build analyses typically do not consider total costs of ownership, and unfortunately decisions are often made by people who are not directly affected by the workflow obstacles or benefits that result from those decisions. We have been developing custom software for clinical use for over a decade, and this article presents our perspective on this practice. A complete analysis of the decision to develop or purchase must ultimately examine how the end result will mesh with the departmental workflow, and custom-developed solutions typically can have the greater positive impact on efficiency and productivity, substantially altering the decision balance sheet. (Full article...)
Recently featured: