Difference between revisions of "Template:Article of the week"
Shawndouglas (talk | contribs) |
Shawndouglas (talk | contribs) (Updated article of the week text.) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig3 Martins BMCMedInfoDecMak2017 17-1.gif|240px]]</div> | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig3 Martins BMCMedInfoDecMak2017 17-1.gif|240px]]</div> | ||
'''"[[Journal: | '''"[[Journal:MASTR-MS: A web-based collaborative laboratory information management system (LIMS) for metabolomics|MASTR-MS: A web-based collaborative laboratory information management system (LIMS) for metabolomics]]"''' | ||
An increasing number of research [[laboratory|laboratories]] and core analytical facilities around the world are developing high throughput metabolomic analytical and data processing pipelines that are capable of handling hundreds to thousands of individual samples per year, often over multiple projects, collaborations and sample types. At present, there are no [[laboratory information management system]]s (LIMS) that are specifically tailored for metabolomics laboratories that are capable of tracking samples and associated metadata from the beginning to the end of an experiment, including data processing and archiving, and which are also suitable for use in large institutional core facilities or multi-laboratory consortia as well as single laboratory environments. | |||
Here we present [[MASTR-MS]], a downloadable and installable LIMS solution that can be deployed either within a single laboratory or used to link workflows across a multisite network.('''[[MASTR-MS: A web-based collaborative laboratory information management system (LIMS) for metabolomics|Full article...]]''')<br /> | |||
<br /> | <br /> | ||
''Recently featured'': | ''Recently featured'': | ||
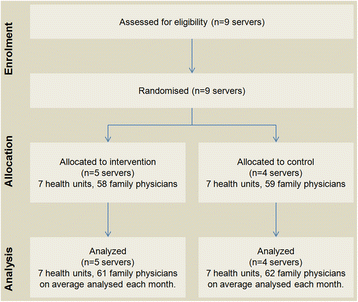
: ▪ [[Journal:The effect of a test ordering software intervention on the prescription of unnecessary laboratory tests - A randomized controlled trial|The effect of a test ordering software intervention on the prescription of unnecessary laboratory tests - A randomized controlled trial]] | |||
: ▪ [[Journal:The state of open-source electronic health record projects: A software anthropology study|The state of open-source electronic health record projects: A software anthropology study]] | : ▪ [[Journal:The state of open-source electronic health record projects: A software anthropology study|The state of open-source electronic health record projects: A software anthropology study]] | ||
: ▪ [[Journal:PCM-SABRE: A platform for benchmarking and comparing outcome prediction methods in precision cancer medicine|PCM-SABRE: A platform for benchmarking and comparing outcome prediction methods in precision cancer medicine]] | : ▪ [[Journal:PCM-SABRE: A platform for benchmarking and comparing outcome prediction methods in precision cancer medicine|PCM-SABRE: A platform for benchmarking and comparing outcome prediction methods in precision cancer medicine]] | ||
Revision as of 17:53, 12 June 2017
"MASTR-MS: A web-based collaborative laboratory information management system (LIMS) for metabolomics"
An increasing number of research laboratories and core analytical facilities around the world are developing high throughput metabolomic analytical and data processing pipelines that are capable of handling hundreds to thousands of individual samples per year, often over multiple projects, collaborations and sample types. At present, there are no laboratory information management systems (LIMS) that are specifically tailored for metabolomics laboratories that are capable of tracking samples and associated metadata from the beginning to the end of an experiment, including data processing and archiving, and which are also suitable for use in large institutional core facilities or multi-laboratory consortia as well as single laboratory environments.
Here we present MASTR-MS, a downloadable and installable LIMS solution that can be deployed either within a single laboratory or used to link workflows across a multisite network.(Full article...)
Recently featured:
- ▪ The effect of a test ordering software intervention on the prescription of unnecessary laboratory tests - A randomized controlled trial
- ▪ The state of open-source electronic health record projects: A software anthropology study
- ▪ PCM-SABRE: A platform for benchmarking and comparing outcome prediction methods in precision cancer medicine