Template:Article of the week
"Recommended versus certified repositories: Mind the gap"
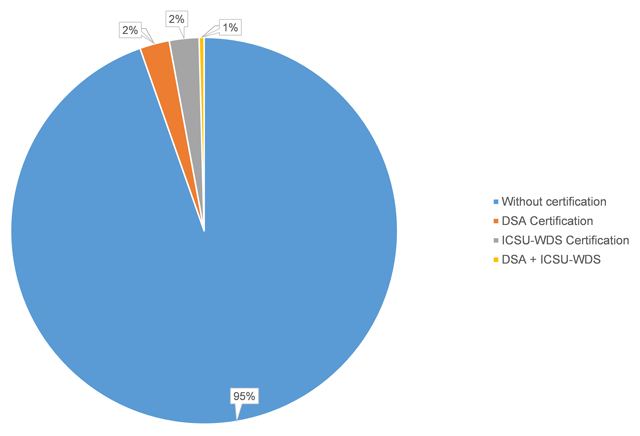
Researchers are increasingly required to make research data publicly available in data repositories. Although several organizations propose criteria to recommend and evaluate the quality of data repositories, there is no consensus of what constitutes a good data repository. In this paper, we investigate, first, which data repositories are recommended by various stakeholders (publishers, funders, and community organizations) and second, which repositories are certified by a number of organizations. We then compare these two lists of repositories, and the criteria for recommendation and certification. We find that criteria used by organizations recommending and certifying repositories are similar, although the certification criteria are generally more detailed. We distill the lists of criteria into seven main categories: “Mission,” “Community/Recognition,” “Legal and Contractual Compliance,” “Access/Accessibility,” “Technical Structure/Interface,” “Retrievability,” and “Preservation.” Although the criteria are similar, the lists of repositories that are recommended by the various agencies are very different. Out of all of the recommended repositories, less than six percent obtained certification. As certification is becoming more important, steps should be taken to decrease this gap between recommended and certified repositories, and ensure that certification standards become applicable, and applied, to the repositories which researchers are currently using. (Full article...)
Recently featured: