Difference between revisions of "User:Shawndouglas/sandbox/sublevel8"
Shawndouglas (talk | contribs) Tag: Reverted |
Shawndouglas (talk | contribs) Tag: Reverted |
||
| Line 12: | Line 12: | ||
<div class="nonumtoc">__TOC__</div> | <div class="nonumtoc">__TOC__</div> | ||
== | ==3. Data management== | ||
Data management in the [[physician office laboratory]] (POL) involves understanding workflows, choosing data management systems, and applying best practices. This chapter talks about the common POL workflow and how data associated with it is better managed with [[informatics]] tools. However, this chapter also recognizes that not all POLs can justify a best-of-class [[laboratory information system]] (LIS) in their operations, noting that hybrid [[electronic health record]] (EHR) systems that include some LIS functionality may make more sense. The concept of middleware is also addressed as another possible means of integrating instrument system data into EHRs, skipping the LIS entirely. | Data management in the [[physician office laboratory]] (POL) involves understanding workflows, choosing data management systems, and applying best practices. This chapter talks about the common POL workflow and how data associated with it is better managed with [[informatics]] tools. However, this chapter also recognizes that not all POLs can justify a best-of-class [[laboratory information system]] (LIS) in their operations, noting that hybrid [[electronic health record]] (EHR) systems that include some LIS functionality may make more sense. The concept of middleware is also addressed as another possible means of integrating instrument system data into EHRs, skipping the LIS entirely. | ||
=== | ===3.1 Workflow and functional requirements=== | ||
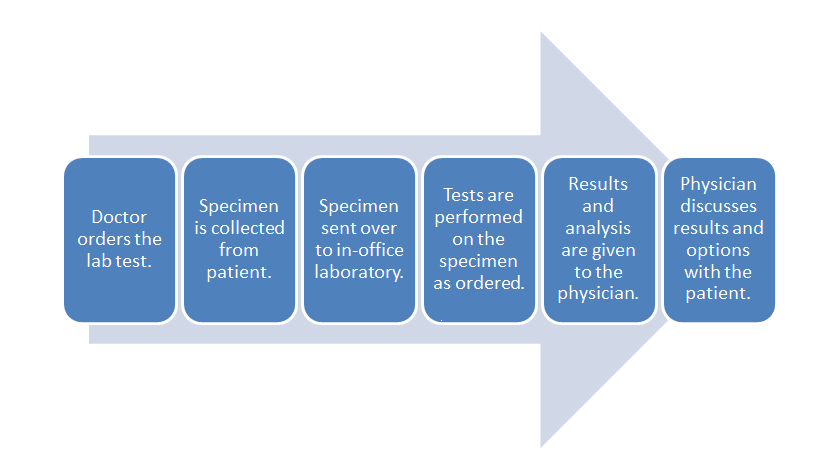
When considering the data management requirements for any business—from a car dealership to a clinical laboratory—the workflow of the business should be one of the primary starting points. This holds equally true for the [[physician office laboratory]] (POL). As such, it's important to note where the POL is located in the broad scope of clinical workflow, which is directly in the analytic stage of the process, with a few pre-analytic and post-analytic tasks occurring directly before and after. As a reminder, here's the POL workflow diagram again from the first chapter of this guide: | When considering the data management requirements for any business—from a car dealership to a clinical laboratory—the workflow of the business should be one of the primary starting points. This holds equally true for the [[physician office laboratory]] (POL). As such, it's important to note where the POL is located in the broad scope of clinical workflow, which is directly in the analytic stage of the process, with a few pre-analytic and post-analytic tasks occurring directly before and after. As a reminder, here's the POL workflow diagram again from the first chapter of this guide: | ||
| Line 45: | Line 45: | ||
If the POL is fully set on acquiring an LIS, it could turn to a more modern approach to requirements specifications in the form of [[LII:LIMSpec 2022 R1|LIMSpec 2022]]. Built from more than 100 regulations, standards, guidance documents, and more, LIMSpec may be a useful component to your LIS acquisition research. A careful review of LIMSpec should provide a clearer picture of the potential requirements of a clinical laboratory. It must be stated, however, that as many POLs are smaller in scale, more than a few statements—such as "The system includes clinical trial management tools"—will be viewed as unnecessary to all but clinical research laboratories. In other words, just because a requirement statement is listed doesn't mean that functionality will be necessary to a POL. | If the POL is fully set on acquiring an LIS, it could turn to a more modern approach to requirements specifications in the form of [[LII:LIMSpec 2022 R1|LIMSpec 2022]]. Built from more than 100 regulations, standards, guidance documents, and more, LIMSpec may be a useful component to your LIS acquisition research. A careful review of LIMSpec should provide a clearer picture of the potential requirements of a clinical laboratory. It must be stated, however, that as many POLs are smaller in scale, more than a few statements—such as "The system includes clinical trial management tools"—will be viewed as unnecessary to all but clinical research laboratories. In other words, just because a requirement statement is listed doesn't mean that functionality will be necessary to a POL. | ||
=== | ===3.2. LIS integration with software and instruments=== | ||
Given that a clinical laboratory's workflow often includes analytical instruments and other software, it makes sense that those tools should be able to [[LIS feature#Instrument interfacing and management|interface]] with a data management system like an LIS. Today's interfaces are generally built on standardized communication tools, including messaging formats like Health Level 7's (HL7's) [[Health Level 7#Fast Healthcare Interoperability Resources (FHIR)|FHIR]].<ref name="Sinard06" /> Arguably, this interoperability may not be a requirement for POLs performing waived testing; CLIA-waived tests don't often require advanced equipment, and the POL may not even need an LIS to manage patient test data. But for those POLs that employ [[laboratory automation]], the ability of clinical analyzers to send their data to a central laboratory information system is important to maintaining a streamlined, efficient workflow. | Given that a clinical laboratory's workflow often includes analytical instruments and other software, it makes sense that those tools should be able to [[LIS feature#Instrument interfacing and management|interface]] with a data management system like an LIS. Today's interfaces are generally built on standardized communication tools, including messaging formats like Health Level 7's (HL7's) [[Health Level 7#Fast Healthcare Interoperability Resources (FHIR)|FHIR]].<ref name="Sinard06" /> Arguably, this interoperability may not be a requirement for POLs performing waived testing; CLIA-waived tests don't often require advanced equipment, and the POL may not even need an LIS to manage patient test data. But for those POLs that employ [[laboratory automation]], the ability of clinical analyzers to send their data to a central laboratory information system is important to maintaining a streamlined, efficient workflow. | ||
The HL7 messaging standards are particularly important to laboratory data management because they define how information is packaged and communicated from one party to another. Such standards set the language, structure, and data types required for seamless integration of various systems and instruments.<ref name="KimCreating05">{{cite web |url=http://www.kathykim.com/sitebuildercontent/sitebuilderfiles/ClinicalDataStandardsInHealthCare.pdf |archiveurl=https://web.archive.org/web/20170114055221/http://www.kathykim.com/sitebuildercontent/sitebuilderfiles/ClinicalDataStandardsInHealthCare.pdf |format=PDF |title=Creating Clinical Data Standards in Health Care: Five Case Studies |author=Kim, K. |publisher=California HealthCare Foundation |date=July 2005 |archivedate=14 January 2017 |accessdate=17 May 2022}}</ref> Over the years, Health Level 7 has described the types of information communicated between such systems in the clinical environment as including "process control and status information for each device or analyzer, [as well as] each specimen, specimen container, and container carrier; information and detailed data related to patients, orders, and results; and information related to specimen flow algorithms and automated decision making."<ref name="HL711">{{cite web |url=http://www.hl7.org/implement/standards/product_brief.cfm?product_id=203 |archiveurl=https://web.archive.org/web/20170628160314/http://www.hl7.org/implement/standards/product_brief.cfm?product_id=203 |title=HL7 version 2.7 standard: Chapter 13 - Clinical laboratory automation |author=Health Level Seven International |date=2011 |archivedate=28 June 2017 |accessdate=17 May 2022}}</ref> | The HL7 messaging standards are particularly important to laboratory data management because they define how information is packaged and communicated from one party to another. Such standards set the language, structure, and data types required for seamless integration of various systems and instruments.<ref name="KimCreating05">{{cite web |url=http://www.kathykim.com/sitebuildercontent/sitebuilderfiles/ClinicalDataStandardsInHealthCare.pdf |archiveurl=https://web.archive.org/web/20170114055221/http://www.kathykim.com/sitebuildercontent/sitebuilderfiles/ClinicalDataStandardsInHealthCare.pdf |format=PDF |title=Creating Clinical Data Standards in Health Care: Five Case Studies |author=Kim, K. |publisher=California HealthCare Foundation |date=July 2005 |archivedate=14 January 2017 |accessdate=17 May 2022}}</ref> Over the years, Health Level 7 has described the types of information communicated between such systems in the clinical environment as including "process control and status information for each device or analyzer, [as well as] each specimen, specimen container, and container carrier; information and detailed data related to patients, orders, and results; and information related to specimen flow algorithms and automated decision making."<ref name="HL711">{{cite web |url=http://www.hl7.org/implement/standards/product_brief.cfm?product_id=203 |archiveurl=https://web.archive.org/web/20170628160314/http://www.hl7.org/implement/standards/product_brief.cfm?product_id=203 |title=HL7 version 2.7 standard: Chapter 13 - Clinical laboratory automation |author=Health Level Seven International |date=2011 |archivedate=28 June 2017 |accessdate=17 May 2022}}</ref> | ||
==== | ====3.2.1 EHR-LIS integration and interfacing==== | ||
Integration of clinical and physician systems usually requires an interface—often referred to as a result interface—which isn't typically turnkey, begetting the need for a comprehensive development plan and implementation process.<ref name="Kasoff12">{{cite web |url=https://www.mlo-online.com/home/article/13004285/connecting-your-lis-and-ehr |title=Connecting your LIS and EHR |author=Kasoff, J. |publisher=Medical Laboratory Observer |date=February 2012 |accessdate=17 May 2022}}</ref> If designed well, the interface allows test orders and results to be transferred back and forth between the two systems, and it permits batch billing and admission/discharge/transfer (ADT) reporting to occur.<ref name="Sinard06" /> | Integration of clinical and physician systems usually requires an interface—often referred to as a result interface—which isn't typically turnkey, begetting the need for a comprehensive development plan and implementation process.<ref name="Kasoff12">{{cite web |url=https://www.mlo-online.com/home/article/13004285/connecting-your-lis-and-ehr |title=Connecting your LIS and EHR |author=Kasoff, J. |publisher=Medical Laboratory Observer |date=February 2012 |accessdate=17 May 2022}}</ref> If designed well, the interface allows test orders and results to be transferred back and forth between the two systems, and it permits batch billing and admission/discharge/transfer (ADT) reporting to occur.<ref name="Sinard06" /> | ||
| Line 153: | Line 153: | ||
Finally, in cases where POL test volumes are low—coming from only one or a few instruments—and an LIS is not required, POL operators may want to simply consider a middleware option that smoothly facilitates the flow of instrument data to the EHR. | Finally, in cases where POL test volumes are low—coming from only one or a few instruments—and an LIS is not required, POL operators may want to simply consider a middleware option that smoothly facilitates the flow of instrument data to the EHR. | ||
=== | ===3.3 Best practices and standard operating procedures=== | ||
[[File:ISO logo.png|right|200px]]It's not enough for physician office staff to choose and implement information management tools in the practice. Like a stethoscope or a diabetes test kit, information management software is a tool requiring knowledge of how to effectively use and manage it in order to achieve the best results; it won't improve patient and test management all on its own. As such, any physician office or POL adding data management tools should also implement a set of best practices that will better guarantee cleaner, more secure patient and test data. Proper health information management (HIM) practices have the potential to yield higher staff productivity levels, "improve reimbursement by helping create quality records that substantiate the care that physicians bill ... [and] reduce denied claims through better documentation."<ref name="HeubuschPhys08">{{cite journal |url=https://library.ahima.org/doc?oid=82949 |title=Physician Practices and Information Management: HIM Professionals Offer Value in Changing Practices |journal=Journal of AHIMA |author=Heubusch, Kevin |volume=79 |issue=8 |pages=18–22 |year=August 2008 |accessdate=17 May 2022}}</ref> | [[File:ISO logo.png|right|200px]]It's not enough for physician office staff to choose and implement information management tools in the practice. Like a stethoscope or a diabetes test kit, information management software is a tool requiring knowledge of how to effectively use and manage it in order to achieve the best results; it won't improve patient and test management all on its own. As such, any physician office or POL adding data management tools should also implement a set of best practices that will better guarantee cleaner, more secure patient and test data. Proper health information management (HIM) practices have the potential to yield higher staff productivity levels, "improve reimbursement by helping create quality records that substantiate the care that physicians bill ... [and] reduce denied claims through better documentation."<ref name="HeubuschPhys08">{{cite journal |url=https://library.ahima.org/doc?oid=82949 |title=Physician Practices and Information Management: HIM Professionals Offer Value in Changing Practices |journal=Journal of AHIMA |author=Heubusch, Kevin |volume=79 |issue=8 |pages=18–22 |year=August 2008 |accessdate=17 May 2022}}</ref> | ||
| Line 178: | Line 178: | ||
* hiring a consultant or part-time HIM professional to fill in knowledge gaps.<ref name="HeubuschPhys08" /> | * hiring a consultant or part-time HIM professional to fill in knowledge gaps.<ref name="HeubuschPhys08" /> | ||
=== | ===3.4 Other workflow requirements=== | ||
For the most part, data management systems such as the LIS and EHR/EMR are designed to handle core workflow functions; the LIS manages specimen receiving, processing, and analysis, while the EHR excels at organizing and presenting patient data. However, technological innovation and demand for more comprehensive tools has driven software developers to add additional functionality beyond the main workflow track, including environmental monitoring, customer management, and billing and revenue management. As such, it's worth taking a brief look at any additional workflow requirements in the POL and how data management tools may or may not address them. | For the most part, data management systems such as the LIS and EHR/EMR are designed to handle core workflow functions; the LIS manages specimen receiving, processing, and analysis, while the EHR excels at organizing and presenting patient data. However, technological innovation and demand for more comprehensive tools has driven software developers to add additional functionality beyond the main workflow track, including environmental monitoring, customer management, and billing and revenue management. As such, it's worth taking a brief look at any additional workflow requirements in the POL and how data management tools may or may not address them. | ||
| Line 203: | Line 203: | ||
==Citation information for this chapter== | ==Citation information for this chapter== | ||
'''Chapter''': | '''Chapter''': 3. Data management | ||
'''Title''': ''The Comprehensive Guide to Physician Office Laboratory Setup and Operation'' | '''Title''': ''The Comprehensive Guide to Physician Office Laboratory Setup and Operation'' | ||
Revision as of 22:03, 31 May 2022
|
|
This is sublevel8 of my sandbox, where I play with features and test MediaWiki code. If you wish to leave a comment for me, please see my discussion page instead. |
Sandbox begins below
3. Data management
Data management in the physician office laboratory (POL) involves understanding workflows, choosing data management systems, and applying best practices. This chapter talks about the common POL workflow and how data associated with it is better managed with informatics tools. However, this chapter also recognizes that not all POLs can justify a best-of-class laboratory information system (LIS) in their operations, noting that hybrid electronic health record (EHR) systems that include some LIS functionality may make more sense. The concept of middleware is also addressed as another possible means of integrating instrument system data into EHRs, skipping the LIS entirely.
3.1 Workflow and functional requirements
When considering the data management requirements for any business—from a car dealership to a clinical laboratory—the workflow of the business should be one of the primary starting points. This holds equally true for the physician office laboratory (POL). As such, it's important to note where the POL is located in the broad scope of clinical workflow, which is directly in the analytic stage of the process, with a few pre-analytic and post-analytic tasks occurring directly before and after. As a reminder, here's the POL workflow diagram again from the first chapter of this guide:
Inside this workflow are various associated activities, largely dependent on the specimen and test type, as well as the Clinical Laboratory Improvement Amendments (CLIA) certificate type the POL holds[1]:
- Specimen collection: The physician, nurse, or office assistant will go through the necessary procedures to collect what is needed for an ordered test. Some specimens such as intravenous blood and urine will require added collection procedures beyond easy-to-collect specimen types such as capillary blood.
- Specimen receiving: In most cases, the specimen doesn't require special transportation because collection is happening at the point of care. Additionally, the specimen may not require much handling, as in the case of collecting capillary blood from the finger bed for a determination of glucose levels via a hand-held meter. More complex specimens not tested at the patient's side will require extra receiving procedures, however, including adding labeling and barcoding, as well as entering the specimen in an information management system.
- Specimen preparation: In a POL where intravenous blood and urine are collected, those specimen types may require extra preparation if not performed in receiving. Blood, for example, may need to be centrifuged and have the serum plasma pipetted out as an aliquot.
- Specimen processing and analysis: In the case of simple-to-use CLIA-waved test devices, the processing and analysis—often qualitative—is performed by the device, producing results in some cases within a few minutes. In the less frequent case of moderate to complex testing in a POL, additional processing such as the addition of a reagent or placement in an analyzer may be required. This culminates in a manual analysis by a pathologist, cytotechnolgist, or other lab personnel, or an automatic analysis by an analyzer.
- Post-processing: This can range from simply disposing of a CLIA-waived test strip properly to reserving a portion of the specimen in storage for a set period of time in case an additional analysis is required.
- Reporting: In many CLIA-waived devices, the reporting process amounts to simply documenting the result of a test in a patient chart. A few waived devices and most moderate to complex analyzers will generate a report of results either digitally or as a printout. Advanced lab testing requiring human analysis may necessitate the manual generation of a report for the ordering physician. In rare cases such as a positive result for HIV, additional reporting requirements to local and state authorities may also be required.[2] Of course, the final results are then related to the patient, and the physician reviews any necessary next steps.
These activities represent the core of what a basic clinical or physician office laboratory will do. However, a bevy of satellite tasks associated with the day-to-day operation of the POL add additional nuances and requirements when considering how to better manage the lab's data and activities. Ordering inventory, scheduling lab appointments, and maintaining CLIA requirements are all examples of such satellite tasks. An analysis of those tasks may in return prompt questions about how their efficiency can be improved, if it all, through an information management system. Additionally, systems may vary in purpose and functionality, requiring comparisons of more than one type of system based on POL requirements. A POL with a CLIA certificate of waiver that performs a handful of capillary blood and urine tests may not be able to justify a full-fledged laboratory information system (LIS) to manage the data associated with those tests. Results may just need to be entered on a patient chart and/or in an electronic health record (EHR). However, a POL certified to do moderate and complex testing and doing it in sufficient volume may need to turn to an in-house LIS.
So far we've said that looking at a business' workflow makes for a nice start to determining its data management needs. This process includes not only looking at core tasks such as sample processing and reporting but also ancillary tasks such as inventory management, payment processing, and performance tracking. What hasn't been said so far is that knowledgeable vendors of information management systems have covered a lot of that ground themselves already, determining common functional requirements of clinical labs and adding corresponding tools to their software; laboratory workflow drives LIS and other information system development.[3] As such, looking at the features of typical clinical data management tools often provides additional insight. However, another more practical approach involves an amalgam of these two methods: developing a comprehensive request for information (RFI) or request for proposal (RFP).
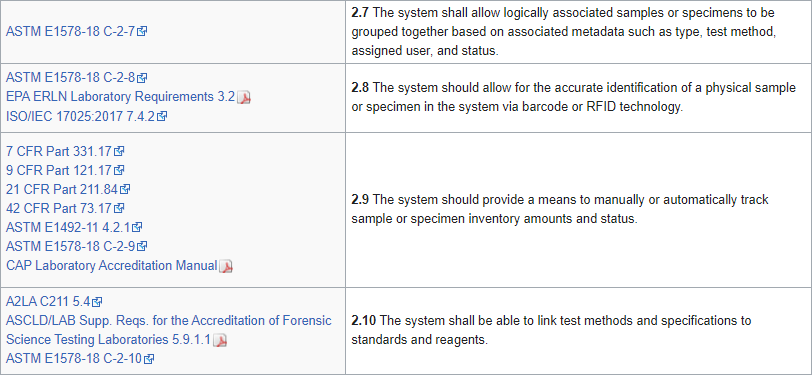
Over the years, several industry-based organizations have created RFI- and RFP-style documents for LIS and laboratory functionality, including the Association for Pathology Informatics (API) and the Healthcare Information and Management Systems Society (HIMSS).[4][5] Those documents contain tens to hundreds of software requirements—which may or may not be organized into a full-fledged specification—based on the operational and administrative needs of a clinical laboratory. An example of requirements statements can be seen in Figure 1:
Figure 1: Sample of laboratory informatics requirements from LIMSpec 2022 R1
While helpful to develop a full list of requirements for your lab, often times such requirements documents turn into a mix of "wishlist" requirements from potential and active clients, as well as regulation-mandated requirements. The wishlist items aren't necessarily ignored by developers, but they do in fact have to be prioritized as "nice to have" or "essential to system operation," or something in between.[6][7][8]
If the POL is fully set on acquiring an LIS, it could turn to a more modern approach to requirements specifications in the form of LIMSpec 2022. Built from more than 100 regulations, standards, guidance documents, and more, LIMSpec may be a useful component to your LIS acquisition research. A careful review of LIMSpec should provide a clearer picture of the potential requirements of a clinical laboratory. It must be stated, however, that as many POLs are smaller in scale, more than a few statements—such as "The system includes clinical trial management tools"—will be viewed as unnecessary to all but clinical research laboratories. In other words, just because a requirement statement is listed doesn't mean that functionality will be necessary to a POL.
3.2. LIS integration with software and instruments
Given that a clinical laboratory's workflow often includes analytical instruments and other software, it makes sense that those tools should be able to interface with a data management system like an LIS. Today's interfaces are generally built on standardized communication tools, including messaging formats like Health Level 7's (HL7's) FHIR.[3] Arguably, this interoperability may not be a requirement for POLs performing waived testing; CLIA-waived tests don't often require advanced equipment, and the POL may not even need an LIS to manage patient test data. But for those POLs that employ laboratory automation, the ability of clinical analyzers to send their data to a central laboratory information system is important to maintaining a streamlined, efficient workflow.
The HL7 messaging standards are particularly important to laboratory data management because they define how information is packaged and communicated from one party to another. Such standards set the language, structure, and data types required for seamless integration of various systems and instruments.[9] Over the years, Health Level 7 has described the types of information communicated between such systems in the clinical environment as including "process control and status information for each device or analyzer, [as well as] each specimen, specimen container, and container carrier; information and detailed data related to patients, orders, and results; and information related to specimen flow algorithms and automated decision making."[10]
3.2.1 EHR-LIS integration and interfacing
Integration of clinical and physician systems usually requires an interface—often referred to as a result interface—which isn't typically turnkey, begetting the need for a comprehensive development plan and implementation process.[11] If designed well, the interface allows test orders and results to be transferred back and forth between the two systems, and it permits batch billing and admission/discharge/transfer (ADT) reporting to occur.[3]
Up until recently, the reality, however, for particularly large laboratories accepting test orders from multiple physician offices has been that tens of different interfaces may need to be developed, one for each different EHR. The end result: an unnecessarily complex process of transferring data between systems.[12] One possible approach to this problem was suggested in 2015 by Dr. Donald M. Voltz: integrate systems through middleware, software similar to a result interface that connects one or more other software applications. From his point of view, a proper middleware solution could connect dozens of EHRs, creating interoperability.[13][14] Though Voltz promoted middleware as a solution to EHR-to-EHR integration, its applicability to integrating an LIS to an EHR is still highly relevant.
In fact, the College of American Pathologists' CAP Today publication reported on the trend of middleware development for POLs in 2011, for not only software-to-software integration but also software-to-instrument integration. "For the right POL, middleware can provide a user-friendly, flexible, cost-effective method for interfacing their IVD [in vitro diagnostics] instruments directly to the EHR without the need for an LIS," said Dawning Technologies' Jay Sax.[15] Today, thanks to wider adoption of HL7, FHIR, POCT1-A, and other standards, more powerful middleware solutions capable of interfacing instruments, EHRs, and even LISs are available to the POL. See Table 1 for a representative sampling of such middleware.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Some software developers have also attempted to simply add LIS functionality to an EHR (EHR-LIS). But this approach has its drawbacks. In 2012, the College of American Pathologists' (CAP's) Diagnostic Intelligence and Health Information Technology (DIHIT) Committee identified several issues with this approach, including[16]:
- a limited feature set, with little incentive to increase functionality;
- a failure to eliminate the need for interfaces with other systems;
- an encounter-specific approach rather than a specimen-centric one; and
- an inability to handle outreach volume and operations.
In fact, a 2022 market report by Signify Research shows that the EHR-LIS has made significant inroads at the expense of the "best of breed" LIS, primarily due to the "comparatively lower price point" and simplification of the IT supply chain. However, Signify also notes that such EHR-LIS hybrids generally come with the some downside, such as "a loss in specialization for workflows and lab types."[17]
Another longstanding complaint about EHRs and the way they interface with an LIS: many don't handle test-related activities well. "[O]nly a handful of EHR products are good at supporting optimal workflow for lab test orders and lab test reporting," according to a 2015 report from Dark Daily.[12] That same report also mentions how when test results are finally transferred back from an LIS to an EHR, the associated report will often end up "in a secondary location within the EHR, in effect separately from the patient-centric screens the physician uses to view all data needed for diagnosis and treatment of a patient," which as a result "may require the staff to print out the lab test results, which defeats the purpose of an electronic interface."[12]
As such, any POL performing sufficient volumes of testing to benefit from using an LIS may also want to consider the costs and drawbacks, if any, of interfacing to their EHR system. In a case where the POL is in a position to consider both an LIS and an EHR at the same time, examine the features and potential integration of those products, and be sure to consider any future potential of integrating your systems with other external data management systems, including another reference laboratory. If considering an EHR that includes some LIS functionality, be sure to clearly identify the functional requirements and thoroughly demo the system to ensure test and reporting workflows make sense.
Finally, in cases where POL test volumes are low—coming from only one or a few instruments—and an LIS is not required, POL operators may want to simply consider a middleware option that smoothly facilitates the flow of instrument data to the EHR.
3.3 Best practices and standard operating procedures
It's not enough for physician office staff to choose and implement information management tools in the practice. Like a stethoscope or a diabetes test kit, information management software is a tool requiring knowledge of how to effectively use and manage it in order to achieve the best results; it won't improve patient and test management all on its own. As such, any physician office or POL adding data management tools should also implement a set of best practices that will better guarantee cleaner, more secure patient and test data. Proper health information management (HIM) practices have the potential to yield higher staff productivity levels, "improve reimbursement by helping create quality records that substantiate the care that physicians bill ... [and] reduce denied claims through better documentation."[18]
But who's going to take responsibility for developing and maintaining information management best practices in the physician office and its associated laboratory? In larger POLs, a HIM professional may be dedicated to practice and data management duties.[18] However, with 70.6 percent of non-exempt POLs in the U.S. running on a CLIA certificate of waiver[19], a majority of POLs tend to be quite small, overseen not by a laboratory director but rather a person operating as the practice manager. That may be an office manager or a physician in the practice, who in reality may not have a lot of experience with information management procedures and technologies. As such, an information management system's ease-of-use and dependability is often viewed as vital to POL staff who are only just beginning to learn how to effectively develop and implement an effective information management plan.
Standards like ISO 15189 Medical laboratories — Requirements for quality and competence suggest the development of standard operating procedures (SOPs) for the laboratory. These SOPs and best practices also apply to the use of information management tools in the POL. As such, it helps to look at the responsibilities that the office manager or physician will have as part of SOP and best practice development. They may[18][20]:
- document and assess POL and practice workflow;
- select and implement an EHR, LIS, and/or middleware;
- create and maintain health records, including transitioning old data to the new system;
- manage the creation and implementation of quality system documentation and training material;
- manage privacy, confidentiality, and sharing policies for test and patient data; and
- manage quality and compliance reporting duties.
These tasks and duties help shape how SOPs for information management are developed. Defining POL and practice workflow before choosing an information management system, for example, helps clarify what system functionality is necessary and how data entry procedures will potentially alter that workflow upon system implementation. Thinking about a practice's existing privacy, confidentiality, sharing, and reporting duties and how they'll be affected by adding information management tools helps drive post-implementation SOP development.
The practice/data manager requiring additional guidance in developing a POL's data management SOPs may turn to other laboratories' existing SOPs. One such example is Johns Hopkins University's Safety Monitoring in International Laboratories (SMILE) program, which offers at least elementary tools towards developing information management policy in the form of an SOP Checklist.[21] (Original .doc archived here) Pages seven and eight offer insights into information management considerations such as patient confidentiality, electronic information access and use, reporting, results modification, communication of changes, and data storage and integrity.
Other options for POL staff to gain insight into SOP development include:
- reviewing the chosen data management system's documentation;
- taking certified courses on health information technology, for example through the American Medical Informatics Association (AMIA)[22] (found here);
- reading implementation stories from other physician offices and POLs.[20][23]; and
- hiring a consultant or part-time HIM professional to fill in knowledge gaps.[18]
3.4 Other workflow requirements
For the most part, data management systems such as the LIS and EHR/EMR are designed to handle core workflow functions; the LIS manages specimen receiving, processing, and analysis, while the EHR excels at organizing and presenting patient data. However, technological innovation and demand for more comprehensive tools has driven software developers to add additional functionality beyond the main workflow track, including environmental monitoring, customer management, and billing and revenue management. As such, it's worth taking a brief look at any additional workflow requirements in the POL and how data management tools may or may not address them.
1. Quality management: In their 2011 manual on quality management systems, the World Health Organization (WHO) states the following about the importance of laboratory quality:
Laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. The laboratory results must be as accurate as possible, all aspects of the laboratory operations must be reliable, and reporting must be timely in order to be useful in a clinical or public health setting.[24]
Given how vital reliably accurate and prompt test results are to the clinical environment, quality management is a necessary ancillary component to the POL's primary workflow. For CLIA-waived POLs, quality management procedures may be relatively simple, focusing on proper sample collection, test procedures, and result documentation. For moderate- and complex-level POLs, quality management procedures and documentation may be much more rigorous. For the POL not using an information management system, quality management comes down to developing standard operating procedures (SOPs) and a means to train staff and evaluate the SOPs' effectiveness. However, an LIS often includes functionality that promotes quality control beyond in-house SOP development and implementation. Quality control tasks can be scheduled and Westgard rules—"[r]ules that are used in conjunction with each other to provide a high level of error detection while reducing the incidence of false rejection"[25]—can be implemented in the LIS' quality control tools. POL staff benefit from receiving alerts on out-of-range values of test results and viewing Levey-Jennings plots which provide a better visual indication of whether or not a laboratory test is working as expected.[26] It can also house corrective action plans and QC task history.[1] Again, however, the need for software-based quality management tools in the POL is going to largely depend on the POL's specimen and test types, as well as the CLIA certificate type the POL holds.
2. Reporting: Reporting represents another ancillary task that comes out of processing patient specimens. And like quality management, the breadth of reporting in a POL is primarily determined by the volume and difficulty of lab testing. Like so many other tasks, the POL that is dealing with low-volume CLIA-waived testing may simply opt to document test result directly to the patient file. However, opportunities exist for even the smallest of POLs to integrate a variety of reporting functions into their workflow, even if it's through a third-party reporting platform. Aside from specimen reports and test results, inventory, billing, workload, profitability, quality control, regulatory, compliance, and epidemiology can all be documented in text, tables, charts, etc. in a variety of digital, printable formats. The reports can then be automatically faxed, e-mailed, or printed, or delivered via an affiliated provider or patient portal.
3. Other laboratory management tasks: Of course, other POL tasks may originate from the main workflow or simply fall under the category of "general laboratory management." Some of those tasks—often able to be managed via an information management system like an LIS, EHR, or practice management suite—include:
- task assignment and management
- demographic management of customers, suppliers, and other outside entities
- inventory management
- creation, modification, and maintenance of training records and materials
- incident and performance tracking
- billing and revenue management
References
- ↑ 1.0 1.1 Laughlin, Sandra; Feldman, Deb (22 October 2015). "The LIS, the healthcare market, and the POL". Medical Laboratory Observer. MLO – NP Communications, LLC. https://www.mlo-online.com/information-technology/lis/article/13008470/the-lis-the-healthcare-market-and-the-pol. Retrieved 17 May 2022.
- ↑ "HIV Disclosure Policies and Procedures". HIV.gov. U.S. Department of Health & Human Services. 15 May 2017. https://www.hiv.gov/hiv-basics/living-well-with-hiv/your-legal-rights/limits-on-confidentiality. Retrieved 17 May 2022.
- ↑ 3.0 3.1 3.2 Sinard, J.H. (2006). Practical pathology informatics: Demystifying informatics for the practicing anatomic pathologist. Springer-Verlag. doi:10.1007/0-387-28058-8. ISBN 9780387280585.
- ↑ "LIS Functionality Assessment Toolkit". Association for Pathology Informatics. 20 September 2013. https://www.pathologyinformatics.org/lis-toolkit. Retrieved 17 May 2022.
- ↑ "RFP Sample Documents". Healthcare Information and Management Systems Society. March 2012. Archived from the original on 02 June 2016. https://web.archive.org/web/20160602193653/http://www.himss.org/resourcelibrary/TopicList.aspx?MetaDataID=565. Retrieved 17 May 2022.
- ↑ Aasem, M.; Ramzan, M.; Jaffar, A. (2010). "Analysis and optimization of software requirements prioritization techniques". Proceedings from the 2010 International Conference on Information and Emerging Technologies: 1–6. doi:10.1109/ICIET.2010.5625687.
- ↑ Hirsch, J. (22 November 2013). "10 Steps To Successful Requirements Gathering". Phase2 Technology, LLC. https://www.phase2technology.com/blog/successful-requirements-gathering. Retrieved 17 May 2022.
- ↑ Burris, E. (2007). "Requirements Specification". CS451R, University of Missouri–Kansas City. University of Missouri–Kansas City. Archived from the original on 24 July 2019. https://web.archive.org/web/20190724173601/http://sce2.umkc.edu/BIT/burrise/pl/requirements/. Retrieved 17 May 2022.
- ↑ Kim, K. (July 2005). "Creating Clinical Data Standards in Health Care: Five Case Studies" (PDF). California HealthCare Foundation. Archived from the original on 14 January 2017. https://web.archive.org/web/20170114055221/http://www.kathykim.com/sitebuildercontent/sitebuilderfiles/ClinicalDataStandardsInHealthCare.pdf. Retrieved 17 May 2022.
- ↑ Health Level Seven International (2011). "HL7 version 2.7 standard: Chapter 13 - Clinical laboratory automation". Archived from the original on 28 June 2017. https://web.archive.org/web/20170628160314/http://www.hl7.org/implement/standards/product_brief.cfm?product_id=203. Retrieved 17 May 2022.
- ↑ Kasoff, J. (February 2012). "Connecting your LIS and EHR". Medical Laboratory Observer. https://www.mlo-online.com/home/article/13004285/connecting-your-lis-and-ehr. Retrieved 17 May 2022.
- ↑ 12.0 12.1 12.2 "How Medical Laboratories Help Physicians Overcome the Failure of Many EHR Systems to Support Effective Lab Test Ordering and Lab Result Reporting". Dark Daily. Dark Intelligence Group, Inc. 30 March 2015. https://www.darkdaily.com/2015/03/30/how-medical-laboratories-help-physicians-overcome-the-failure-of-many-ehr-systems-to-support-effective-lab-test-ordering-and-lab-result-reporting-330/. Retrieved 17 May 2022.
- ↑ Voltz, Donald M. (May 2015). "Connecting the Disparate: Middleware’s Role in Solving Healthcare’s EHR Interoperability Problems". Journal of AHIMA 86 (5): 28–33. https://library.ahima.org/doc?oid=107645. Retrieved 17 May 2022.
- ↑ Voltz, Donald M.; Tran, Thanh (2 June 2015). "Can a Middleware Prescription Cure Healthcare’s EHR Interoperability Disorder?". Electronic Health Reporter. millerrupp. https://electronichealthreporter.com/can-a-middleware-prescription-cure-healthcares-ehr-interoperability-disorder/. Retrieved 17 May 2022.
- ↑ Wagner, Karen L. (March 2011). "Middleware to ‘littleware’: vendors catering to smaller labs" (PDF). CAP Today. College of American Pathologists. http://www.captodayonline.com/Archives/0311/0310_CAPTODAY_MiddlewareSystemsGuide.pdf. Retrieved 17 May 2022.
- ↑ Sinard, John H. (2012). "Pathology and the LIS in the Era of the EHR" (PDF). College of American Pathologists. Archived from the original on 29 August 2015. https://web.archive.org/web/20150829164712/http://www.pathologyinformatics.org/sites/default/files/Sinard_CAP%20DIHIT%20Committee_API_SS_2012.pdf. Retrieved 17 May 2022.
- ↑ Fitt, I. (26 May 2022). "Why BoB LIS vendors might yet survive EHR’s invasion". Signify Research. https://www.signifyresearch.net/healthcare-it/why-bob-lis-vendors-might-yet-survive-ehrs-invasion/. Retrieved 30 May 2022.
- ↑ 18.0 18.1 18.2 18.3 Heubusch, Kevin (August 2008). "Physician Practices and Information Management: HIM Professionals Offer Value in Changing Practices". Journal of AHIMA 79 (8): 18–22. https://library.ahima.org/doc?oid=82949. Retrieved 17 May 2022.
- ↑ Centers for Medicare and Medicaid Services, Division of Laboratory Services (May 2022). "Enrollment, CLIA exempt states, and certification of accreditation by organization" (PDF). https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/Downloads/statupda.pdf. Retrieved 17 May 2022.
- ↑ 20.0 20.1 Smith, Paul D. (May 2003). "Implementing an EMR System: One Clinic’s Experience". Family Practice Management 10 (5): 37–42. http://www.aafp.org/fpm/2003/0500/p37.html. Retrieved 17 May 2022.
- ↑ Madden, Jaclyn; Hanes, Heidi (ed.) (18 January 2013). "Checklist for Site SOP Required Elements: Quality Management Plan" (DOC). Johns Hopkins University. Archived from the original on 05 January 2016. https://web.archive.org/web/20160105213800/http://www.niaid.nih.gov/labsandresources/resources/daidsclinrsrch/documents/labstudydata.doc. Retrieved 17 May 2022.
- ↑ "AMIA Health Informatics Essentials: Health Information Systems". American Medical Informatics Association. https://amia.org/education-events/education-catalog/amia-health-informatics-essentials-health-information-systems. Retrieved 17 May 2022.
- ↑ Congdon, Ken (2 October 2012). "The Reality Of EHR Use In The Physicians Practice". Health IT Outcomes. Jameson Publishing. https://www.healthitoutcomes.com/doc/the-reality-of-ehr-use-in-the-physicians-practice-0001. Retrieved 17 May 2022.
- ↑ World Health Organization (2011) (PDF). Laboratory Quality Management System Handbook (1.1 ed.). Geneva, Switzerland: World Health Organization. ISBN 9789241548274. https://apps.who.int/iris/bitstream/handle/10665/44665/9789241548274_eng.pdf. Retrieved 17 May 2022.
- ↑ Dawson, Sarah (16 February 2005). "Westgard Rules: The Nitty Gritty of Quality Control" (PDF). HPTN Central Lab. Archived from the original on 10 October 2015. https://web.archive.org/web/20151010050214/http://www.hptn.org/Web%20Documents/HPTNMeeting2005/WestgardPresentation02_16_05.pdf. Retrieved 17 May 2022.
- ↑ Moumtzoglou, Anastasius; Kastania, Anastasia; Archondakis, Stavros (2014). Laboratory Management Information Systems: Current Requirements and Future Perspectives. Hershey, PA: IGI Global. p. 285. ISBN 9781466663213. https://books.google.com/books?id=lhqXBQAAQBAJ&pg=PA285.
Citation information for this chapter
Chapter: 3. Data management
Title: The Comprehensive Guide to Physician Office Laboratory Setup and Operation
Author for citation: Shawn E. Douglas
License for content: Creative Commons Attribution-ShareAlike 4.0 International
Publication date: June 2022