Difference between revisions of "Glaucoma valve"
(Transcluded, per John) |
m (→Notes: Added cats) |
||
| Line 2: | Line 2: | ||
==Notes== | ==Notes== | ||
This article is a direct transclusion of [https://en.wikipedia.org/wiki/Glaucoma_valve the Wikipedia article] and therefore may not meet the same editing standards as LIMSwiki. | This article is a direct transclusion of [https://en.wikipedia.org/wiki/Glaucoma_valve the Wikipedia article] and therefore may not meet the same editing standards as LIMSwiki. | ||
<!---Place all category tags here--> | |||
[[Category:Articles transcluded from other wikis]] | |||
[[Category:Implants (medicine)]] | |||
[[Category:Medical devices]] | |||
Latest revision as of 22:48, 22 February 2016
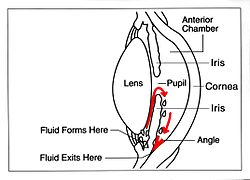
A glaucoma valve is a medical shunt used in the treatment of glaucoma to reduce the eye's intraocular pressure (IOP).
Mechanism
The device works by bypassing the trabecular meshwork and redirecting the outflow of aqueous humour through a small tube into an outlet chamber or bleb. The IOP generally decreases from around 33 to 10 mmHg by removing aqueous on average 2.75 microliters/min.[1]
Types
The first glaucoma drainage implant was developed in 1966.[2] Following on the success of the Molteno implant, several varieties of device have been developed from the original, the Baerveldt tube shunt, or the valved implants, such as the Ahmed glaucoma valve implant and the later generation pressure ridge Molteno implants. These are indicated for glaucoma patients not responding to maximal medical therapy, with previous failed guarded filtering surgery (trabeculectomy). The flow tube is inserted into the anterior chamber of the eye and the plate is implanted underneath the conjunctiva to allow flow of aqueous fluid out of the eye into a chamber called a bleb.
The ExPress Mini Shunt is a newer, non-valved device that was originally designed to provide a direct conduit from the anterior chamber to the sub-conjunctival space or bleb. In this position it was unstable and tended to erode through the conjunctiva. Now the more common use is as a modification of the trabeculectomy procedure, placed under a scleral flap, replacing the sclerostomy step (see trabeculectomy).
In comparison to the glaucoma drainage devices that use an ab externo procedure, ab interno implants, such as the Xen Gel Stent, are transscleral implants to channel aqueous humor into the non-dissected Tenon's space, creating a subconjunctival drainage area similar to a bleb.[3][4] The implants are transscleral and different from more other ab interno implants that do not create a transscleral drainage, such as iStent, CyPass, or Hydrus.[5]
A Cochrane Review of various aqueous shunts and modifications found that the Baerveldt implant may result in lower IOP than the Ahmed implant, but it was unclear if the difference in IOP reduction was clinically significant.[6] The review suggests that practitioners should operate with the devices that they are most comfortable with, and have the most experience using.
Indications
The glaucoma valve implant is indicated for glaucoma patients not responding to maximal medical therapy, with previous failed guarded filtering surgery (trabeculectomy) or in cases where conventional drainage surgery is unlikely to succeed. Common situations where the use of a glaucoma implant as a primary procedure is indicated include
- Neovascular glaucoma – glaucoma associated with vascular disease of the eye (often diabetes).
- Cases of Uveitis – acute or chronic inflammation of the eye.
- Traumatic glaucoma – glaucoma associated with injury to the eye.
- Silicone glaucoma – glaucoma due to Silicone used to repair a detached retina.
- Infantile/Juvenile glaucoma – often associated with developmental defects of the eye.[7]
Surgical technique
The flow tube is inserted into the anterior chamber of the eye and the plate is implanted underneath the conjunctiva to allow flow of aqueous fluid out of the eye.
- The first-generation Molteno and other non-valved implants sometimes require the ligation of the tube until the bleb formed is mildly fibrosed and water-tight.[8] This is done to reduce postoperative hypotony – sudden drops in postoperative intraocular pressure (IOP).
- Valved implants such as the Ahmed glaucoma valve attempt to control postoperative hypotony by using a mechanical valve. Studies show that in severe cases of glaucoma, double plate Molteno implants are associated with lower mean IOP in the long term compared to the Ahmed glaucoma valve.[9]
- Second and third generation Molteno implants incorporate a biological valve and studies show considerable improvement in postoperative outcome over the older style Ahmed and Molteno implants.
- Baervedlt shunts (non-valved) have been shown to have lower rates of surgical failure than Ahmed shunts (valved),[10][11] this have may be related to the high rate of valve failure, or to the larger plate surface of the Baerveldt shunt.
Complications
The majority of complications occur shortly after surgery. These are generally related to high pressure (due to inflammation following surgery) or low pressure (too much aqueous flow through the tube). Periods of low pressure which are more associated with non-valved shunts, can cause retinal detachments, hypotony maculopathy or haemorrhages. Periods of high pressures, which are more associated with valved shunts, are detrimental to the optic nerve. Long term complications of this surgery include diplopia and corneal oedema.
There are also device related complications, which will require surgical revision. For example, erosion, where the conjunctiva erodes over the shunt leaving it exposed, the condition of which may be revised or prevented in advance by the use of amniotic membrane,[12] The ologen collagen matrix facilitates tissue regeneration and its application over the site of device implantation can strengthen tissue recovery, reducing possibility of erosion.[13][14][12][15][16]
When the device malfunctions it may need to be replaced. Possible scenarios include blockage, where a particle becomes logged in the tube line blocking flow; retraction, where the tube line slips out of correct position such that flow is inhibited or halted; valve failure, where the valve stops working blocking flow completely.
Surgical failure occurs due to the ongoing scarring over the conjunctival dissipation segment of the shunt may become too thick for the aqueous humor to filter through. This may require preventive measures using anti-fibrotic medication like 5-fluorouracil (5FU) or Mitomycin-C (during the procedure), or creating a necessity for revision surgery with the sole or combinative use of biodegradable spacer or collagen matrix implant.[6] A Cochrane Review comparing aqueous shunt surgery with and without Mitomycin-C did not find benefit or harm associated with the intervention.[17]
See also
References
- ^ Brubaker, Richard F. (1991-12-01). "Flow of Aqueous Humor in Humans [The Friedenwald Lecture]". Investigative Ophthalmology & Visual Science. 32 (13): 3145–66. PMID 1748546. Archived from the original on 2020-05-21. Retrieved 2012-10-30.
- ^ "Archived copy" (PDF). Archived from the original (PDF) on 2015-01-13. Retrieved 2012-05-15.
{{cite web}}: CS1 maint: archived copy as title (link)[full citation needed] - ^ Lewis RA (Aug 2014). "Ab interno approach to the subconjunctival space using a collagen glaucoma stent". J Cataract Refract Surg. 40 (8): 1301–6. doi:10.1016/j.jcrs.2014.01.032. PMID 24943904.
- ^ "Xen Gel Stent". AqueSys. Archived from the original on 29 June 2015. Retrieved 27 June 2015.
- ^ "Advances in Glaucoma Filtration Surgery". Glaucoma Today. Archived from the original on 29 June 2015. Retrieved 27 June 2015.
- ^ a b Tseng VL, Coleman AL, Chang MY, Caprioli J (2017). "Aqueous shunts for glaucoma". Cochrane Database Syst Rev. 2017 (7) CD004918. doi:10.1002/14651858.CD004918.pub3. PMC 5580949. PMID 28750481.
- ^ Oscar, Albis-Donado; Gil-Carrasco, Gil-Carrasco; Romero-Quijada, Romero-Quijada; Thomas, Thomas (2010). "Evaluation of ahmed glaucoma valve implantation through a needle-generated scleral tunnel in Mexican children with glaucoma". Indian Journal of Ophthalmology. 58 (5): 365–73. doi:10.4103/0301-4738.67039. PMC 2992909. PMID 20689189.
- ^ Molteno, AC; Polkinghorne, PJ; Bowbyes, JA (1986). "The vicryl tie technique for inserting a draining implant in the treatment of secondary glaucoma". Australian and New Zealand Journal of Ophthalmology. 14 (4): 343–54. doi:10.1111/j.1442-9071.1986.tb00470.x. PMID 3814422.
- ^ Ayyala, RS; Zurakowski, D; Monshizadeh, R; Hong, CH; Richards, D; Layden, WE; Hutchinson, BT; Bellows, AR (2002). "Comparison of double-plate Molteno and Ahmed glaucoma valve in patients with advanced uncontrolled glaucoma". Ophthalmic Surgery and Lasers. 33 (2): 94–101. doi:10.3928/1542-8877-20020301-04. PMID 11942556.
- ^ Budenz, DL; Barton, K; Feuer, WJ; Schiffman, J; Costa, VP; Godfrey, DG; Buys, YM; Ahmed Baerveldt Comparison Study Group (2011). "Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up". Ophthalmology. 118 (3): 443–52. doi:10.1016/j.ophtha.2010.07.016. PMC 3020266. PMID 20932583.
- ^ Christakis, PG; Kalenak, JW; Zurakowski, D; Tsai, JC; Kammer, JA; Harasymowycz, PJ; Ahmed, II (2011). "The Ahmed Versus Baerveldt study: One-year treatment outcomes". Ophthalmology. 118 (11): 2180–9. doi:10.1016/j.ophtha.2011.05.004. PMID 21889801.
- ^ a b Rosentreter, A; Schield AM; Dinslage S; Dietlein TS (2012). "Biodegradable implant for tissue repair after glaucoma drainage device surgery". J Glaucoma. 21 (2): 76–8. doi:10.1097/IJG.0b013e3182027ab0. PMID 21278584. S2CID 40206358.
- ^ Rho, S; Sung Y; Ma KT; Rho SH; Kim CY (2015). "Bleb Analysis and Short-Term Results of Biodegradable Collagen Matrix-Augmented Ahmed Glaucoma Valve Implantation: 6-Month Follow-up". Invest Ophthalmol Vis Sci. 56 (10): 5896–903. doi:10.1167/iovs.15-17480. PMID 26348639.
- ^ Johnson, MS; Sarkisian SR Jr. (2014). "Using a collagen matrix implant (Ologen) versus mitomycin-C as a wound healing modulator in trabeculectomy with the Ex-PRESS mini glaucoma device: a 12-month retrospective review". J Glaucoma. 23 (9): 649–52. doi:10.1097/IJG.0000000000000018. PMID 24240882. S2CID 45334774.
- ^ Rosentreter, A; Mellein AC; Konen WW; Dietlein TS (2010). "Capsule excision and Ologen™ implantation for revision after glaucoma drainage device surgery". Graefes Arch Clin Exp Ophthalmol. 248 (9): 1319–24. doi:10.1007/s00417-010-1385-y. PMID 20405139. S2CID 10384646.
- ^ Aptel, F; Dumas S; Denis P (2009). "Ultrasound biomicroscopy and optical coherence tomography imaging of filtering blebs after deep sclerectomy with new collagen implant". Eur J Ophthalmol. 19 (2): 223–30. doi:10.1177/112067210901900208. PMID 19253238. S2CID 22594085.
- ^ Foo VH, Htoon HM, Welsbie DS, Perera SA (2019). "Aqueous shunts with mitomycin C versus aqueous shunts alone for glaucoma". Cochrane Database Syst Rev. 2019 (4) CD011875. doi:10.1002/14651858.CD011875.pub2. PMC 6472957. PMID 30999387.
External links
Notes
This article is a direct transclusion of the Wikipedia article and therefore may not meet the same editing standards as LIMSwiki.









