LII:The Comprehensive Guide to Physician Office Laboratory Setup and Operation/Data management
Data management in the physician office laboratory (POL) involves understanding workflows, choosing data management systems (like LIS or ELN), and applying best practices. This chapter talks about the common workflow and how data associated with it is better managed with informatics tools.
This fourth chapter on the topic of data management has seven sections.
4. Data Management
Functional requirements
When considering the data management requirements for any business — from a car dealership to a clinical laboratory — the workflow of the business should be one of the primary starting points. This holds equally true for the physician office laboratory (POL). As such it's important to note where the POL is located in the broad scope of clinical workflow, which is directly in the analytic stage of the process, with a few pre-analytic and post-analytic tasks occurring directly before and after. As a reminder, here's the POL workflow diagram again from part one of this guide:
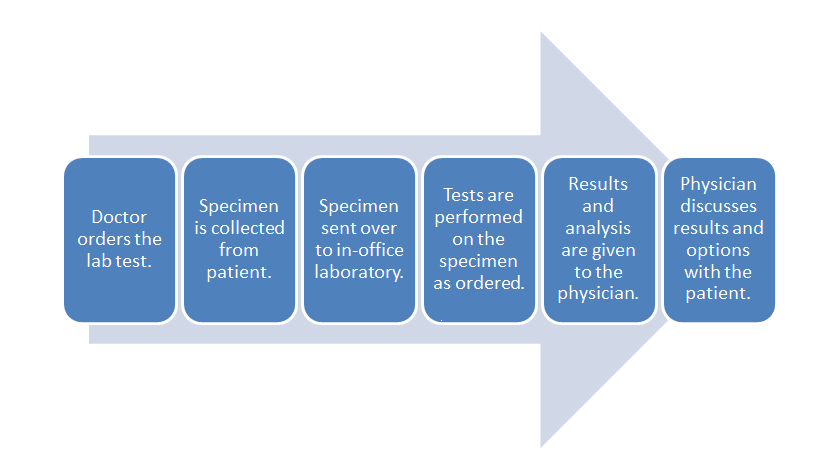
Inside this workflow are various associated activities, largely dependent on the specimen and test type as well as the CLIA certificate type the POL holds:
- Specimen collection: The physician, nurse, or office assistant will go through the necessary procedures to collect what is needed for an ordered test. Some specimens such as intravenous blood and urine will require added collection procedures beyond easy-to-collect specimen types such as capillary blood.
- Specimen receiving: In most cases the specimen doesn't require special transportation because collection is happening at the point of care. Additionally, the specimen may not require much handling, as in the case of collecting capillary blood from the finger bed for a determination of glucose levels via a hand-held meter. More complex samples not tested at the patient's side will require extra receiving procedures, however, including adding labeling and barcoding as well as entering the specimen in a data management system.
- Specimen preparation: In a POL where intravenous blood and urine are collected, those specimen types may require extra preparation if not performed in receiving. Blood, for example, may need to be centrifuged and have the serum plasma pipetted out as an aliquot.
- Specimen processing and analysis: In the case of simple-to-used CLIA-waved test devices, the processing and analysis — often qualitative — is performed by the device, producing results in some cases within a few minutes. In the less frequent case of moderate to complex testing in a POL, additional processing such as the addition of a reagent or placement in an analyzer. This culminates in a manual analysis by a pathologist, cytotechnolgist, or other lab personnel or an automatic analysis by an analyzer.
- Post-processing: This can range from simply disposing of a CLIA-waived test strip properly to reserving a portion of the specimen in storage for a set period of time in case an additional analysis is required.
- Reporting: In many CLIA-waived devices reporting amounts to simply documenting the result of a test in a patient chart. A few waived devices and most moderate to complex analyzers will generate a report of results either digitally or as a printout. Advanced lab testing requiring human analysis may require the manual generation of a report for the office physician. In rare cases such as a positive result for HIV, additional reporting requirements to local and state authorities may be required.[1] Of course, the final results are then related to the patient, and the physician reviews any necessary next steps.
These activities represent the core of what a basic clinical or physician office laboratory will do. However, a bevy of satellite tasks associated with the day-to-day operation of the POL add additional nuances and requirements when considering how to better manage the lab's data and activities. Ordering inventory, scheduling lab appointments, and maintaining CLIA requirements are all examples of such satellite tasks. An analysis of those tasks may in return promps questions about how more efficient they can be made, if it all, through a data management system. Additionally, systems may vary in purpose and functionality, requiring comparisons of more than one type of system based on POL requirements. A POL with a CLIA certificate of waiver that performs a handful of capillary blood and urine tests may not be able to justify a full-fledged laboratory information system (LIS) to manage the data associated with those tests. Results may just need to be entered on a patient chart and/or in an electronic health record (EHR). However, a POL certified to do moderate and complex testing and doing it in sufficient volume may need to turn to an in-house LIS.
So far we've said that looking at a business' workflow makes for a nice start to determining its data management needs. This process includes not only looking at core tasks such as sample processing and reporting but also ancillary tasks such as inventory management, payment processing, and performance tracking. What hasn't been said so far is that competent vendors of data management systems have covered a lot of that ground themselves, determining common functional requirements of clinical labs and adding corresponding tools to their software.
The full set of 320 LIS requirements is included as an addendum to this guide. You can access it directly here or in the Additional Resources section of the guide.
Workflow and integration (EHR, HIS)
Workflow and integration instruments
LIS as a center of workflow/full office integration
LIS relationship to other IT systems
Best practices and standard operating procedures
Other workflow requirements
References
- ↑ "Legal Disclosure". AIDS.gov. U.S. Department of Health & Human Services. 1 June 2012. https://www.aids.gov/hiv-aids-basics/just-diagnosed-with-hiv-aids/your-legal-rights/legal-disclosure/. Retrieved 17 August 2015.









