Difference between revisions of "User:Shawndouglas/sandbox/sublevel4"
From LIMSWiki
< User:Shawndouglas | sandbox
Jump to navigationJump to searchShawndouglas (talk | contribs) |
Shawndouglas (talk | contribs) |
||
| Line 1: | Line 1: | ||
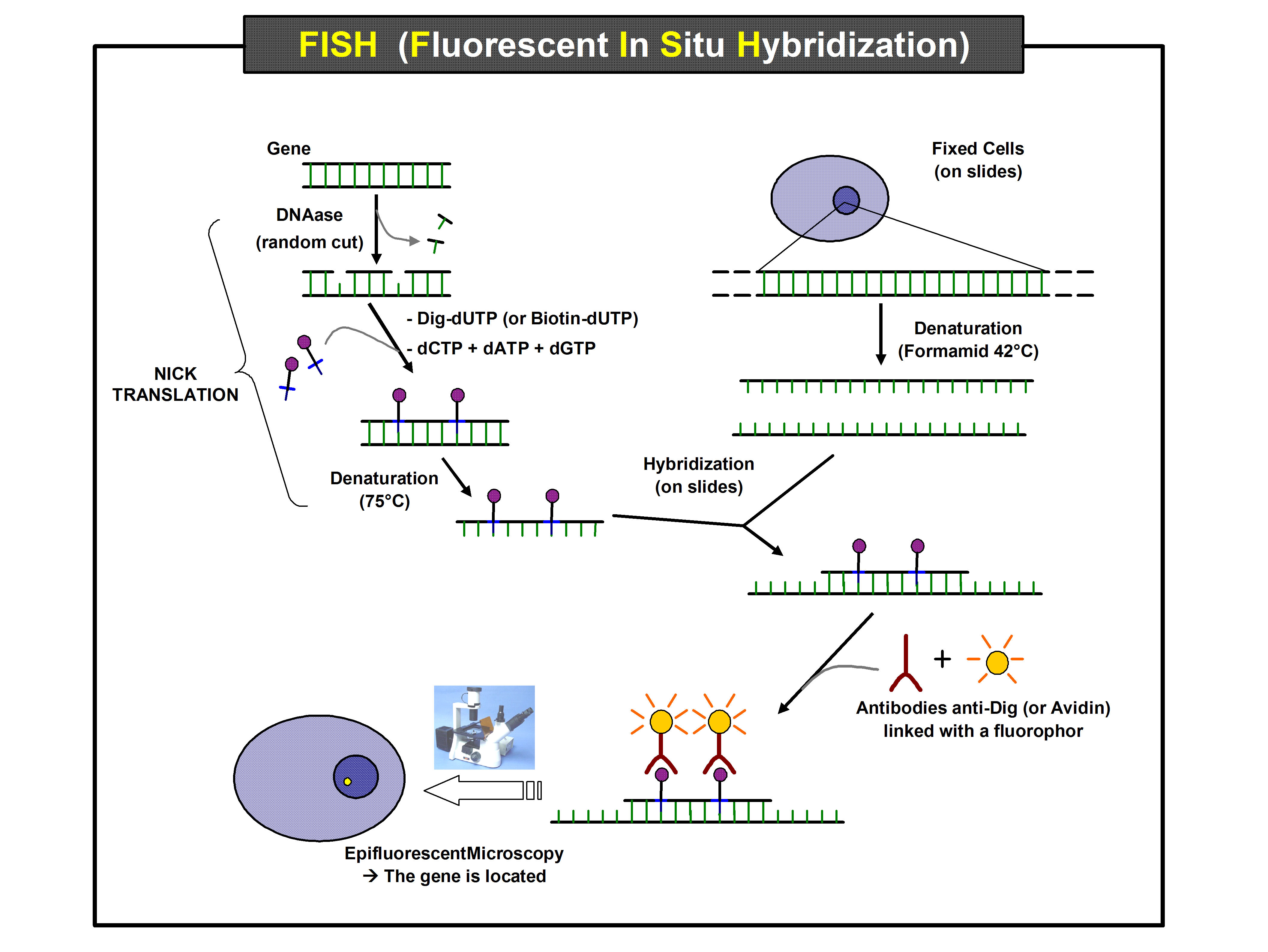
[[File: | [[File:FISH (Fluorescent In Situ Hybridization).jpg|right|550px]]The molecular diagnostics lab will use one or more techniques (e.g., PCR, DNA sequencing, microarrays, gene expression profiling and cytogenetics) in their workflow, and the LIMS used in molecular diagnostics ideally will address those workflow needs. This is especially true for cytogenetics labs, which use specialty techniques like chromosome analysis or karyotyping, fluorescence ''in situ'' hybridization (FISH) and microarray-based assays such as comparative genomic hybridization.<ref name="AACCGenetic19">{{cite web |url=https://www.testing.com/genetic-testing-techniques/ |title=Genetic Testing Techniques |work=Testing.com |publisher=OneCare Media |date=09 November 2021 |accessdate=18 November 2021}}</ref><ref name="MayoClinicCyto">{{cite web |url=https://www.mayoclinic.org/departments-centers/laboratory-medicine-pathology/overview/specialty-groups/laboratory-genetics/cytogenetics-laboratory |title=Cytogenetics Laboratory |work=Departments and Centers: Laboratory Medicine and Pathology |publisher=Mayo Clinic |accessdate=18 November 2021}}</ref><ref name="YaleCyto">{{cite web |url=https://medicine.yale.edu/lab/cytogenetics/testing/ |title=Cytogenetics Lab Tests |work=Cytogenetics Lab |publisher=Yale School of Medicine |accessdate=18 November 2021}}</ref> | ||
In addition to the essential features of a standard LIMS, the molecular diagnostics and cytogenetics lab will also be looking for a system that can (or allows users to)<ref name="SunquestMitogen20">{{cite web |url=https://www.sunquestinfo.com/software-and-services/lims/ |title=Sunquest Mitogen LIMS |publisher=Sunquest Information Systems, Inc |date=2021 |accessdate=18 November 2021}}</ref><ref name="XifinMolec20">{{cite web |url=https://www.xifin.com/industry-solutions/laboratory/molecular-diagnostics |title=Molecular Diagnostics |publisher=XIFIN, Inc |date=2021 |accessdate=18 November 2021}}</ref><ref name="PsycheNucleoLIS20">{{cite web |url=https://psychesystems.com/enterprise-laboratory-information-software/nucleolis-molecular-lab-testing-software/ |title=NucleoLIS - Flexible & Modern LIS |publisher=Psyche Systems |date=2021 |accessdate=18 November 2021}}</ref><ref name="MyersLab18">{{cite journal |title=Laboratory Information Systems and Instrument Software Lack Basic Functionality for Molecular Laboratories |journal=Journal of Molecular Diagnostics |author=Myers, C.; Swadley, M.; Carter, A.B. |volume=20 |issue=5 |pages=591–99 |year=2018 |doi=10.1016/j.jmoldx.2018.05.011}}</ref>: | |||
* Manage sample collection kits. | |||
* Manage informed consent documentation. | |||
* Provide customized workflows for molecular and next-generation sequencing (NGS) testing. | |||
* Track specimen and aliquot lineage for cell lines, tissues, slides, etc. | |||
* Track nucleic acid quantity and quality of specimens. | |||
* Support a wide array of molecular testing and associated data fields, including biochemical and molecular genetics, carrier screening, immunology, molecular profiling, prenatal and newborn testing, and pharmacogenetics. | |||
* Provide custom workflows for FISH, PCR, gel electrophoresis, cytogenetics, DNA sequencing and more. | |||
* | * Support specialty testing reimbursement and other revenue management unique to this lab type. | ||
* Support single sign-on with imaging platforms. | |||
* Provide color coding for turn-around time and other testing statuses. | |||
* Track | * Provide cleanly formatted rich-text reports customized for molecular diagnostics. | ||
* Support | |||
* Provide | |||
* | |||
* Support | |||
* | |||
* Provide | |||
==References== | ==References== | ||
{{Reflist|colwidth=30em}} | {{Reflist|colwidth=30em}} | ||
Revision as of 18:35, 9 March 2022
The molecular diagnostics lab will use one or more techniques (e.g., PCR, DNA sequencing, microarrays, gene expression profiling and cytogenetics) in their workflow, and the LIMS used in molecular diagnostics ideally will address those workflow needs. This is especially true for cytogenetics labs, which use specialty techniques like chromosome analysis or karyotyping, fluorescence in situ hybridization (FISH) and microarray-based assays such as comparative genomic hybridization.[1][2][3]
In addition to the essential features of a standard LIMS, the molecular diagnostics and cytogenetics lab will also be looking for a system that can (or allows users to)[4][5][6][7]:
- Manage sample collection kits.
- Manage informed consent documentation.
- Provide customized workflows for molecular and next-generation sequencing (NGS) testing.
- Track specimen and aliquot lineage for cell lines, tissues, slides, etc.
- Track nucleic acid quantity and quality of specimens.
- Support a wide array of molecular testing and associated data fields, including biochemical and molecular genetics, carrier screening, immunology, molecular profiling, prenatal and newborn testing, and pharmacogenetics.
- Provide custom workflows for FISH, PCR, gel electrophoresis, cytogenetics, DNA sequencing and more.
- Support specialty testing reimbursement and other revenue management unique to this lab type.
- Support single sign-on with imaging platforms.
- Provide color coding for turn-around time and other testing statuses.
- Provide cleanly formatted rich-text reports customized for molecular diagnostics.
References
- ↑ "Genetic Testing Techniques". Testing.com. OneCare Media. 9 November 2021. https://www.testing.com/genetic-testing-techniques/. Retrieved 18 November 2021.
- ↑ "Cytogenetics Laboratory". Departments and Centers: Laboratory Medicine and Pathology. Mayo Clinic. https://www.mayoclinic.org/departments-centers/laboratory-medicine-pathology/overview/specialty-groups/laboratory-genetics/cytogenetics-laboratory. Retrieved 18 November 2021.
- ↑ "Cytogenetics Lab Tests". Cytogenetics Lab. Yale School of Medicine. https://medicine.yale.edu/lab/cytogenetics/testing/. Retrieved 18 November 2021.
- ↑ "Sunquest Mitogen LIMS". Sunquest Information Systems, Inc. 2021. https://www.sunquestinfo.com/software-and-services/lims/. Retrieved 18 November 2021.
- ↑ "Molecular Diagnostics". XIFIN, Inc. 2021. https://www.xifin.com/industry-solutions/laboratory/molecular-diagnostics. Retrieved 18 November 2021.
- ↑ "NucleoLIS - Flexible & Modern LIS". Psyche Systems. 2021. https://psychesystems.com/enterprise-laboratory-information-software/nucleolis-molecular-lab-testing-software/. Retrieved 18 November 2021.
- ↑ Myers, C.; Swadley, M.; Carter, A.B. (2018). "Laboratory Information Systems and Instrument Software Lack Basic Functionality for Molecular Laboratories". Journal of Molecular Diagnostics 20 (5): 591–99. doi:10.1016/j.jmoldx.2018.05.011.