File:Fig4 Goldberg mBio2015 6-6.jpg
Original file (750 × 610 pixels, file size: 94 KB, MIME type: image/jpeg)
Summary
| Description |
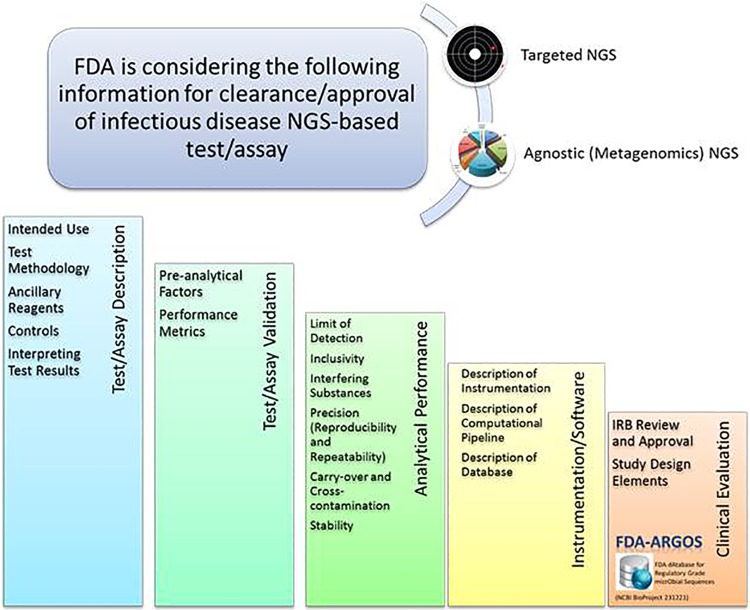
Figure 4. he FDA is considering the following information for the clearance/approval of an infectious disease NGS-based test/assay. The FDA presubmission process can be utilized for outstanding questions and to request additional information while policy is still being developed (26). IRB, institutional review board. |
|---|---|
| Source |
Goldberg, Brittany; Sichtig, Heike; Geyer, Chelsie; Ledeboer, Nathan; Weinstock, George M. (2015). "Making the leap from research laboratory to clinic: Challenges and opportunities for next-generation sequencing in infectious disease diagnostics". mBio 6 (6): e01888-15. doi:10.1128/mBio.01888-15. PMC PMC4669390. PMID 26646014. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4669390. |
| Date |
2016 |
| Author |
Goldberg, Brittany; Sichtig, Heike; Geyer, Chelsie; Ledeboer, Nathan; Weinstock, George M. |
| Permission (Reusing this file) |
Creative Commons Attribution-Noncommercial-ShareAlike 3.0 Unported |
| Other versions |
Licensing
|
|
This work is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. |
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 17:37, 20 September 2016 |  | 750 × 610 (94 KB) | Shawndouglas (talk | contribs) |
You cannot overwrite this file.









