File:Fig5 Smith PracLabMed2024 InPress.jpg
Original file (2,764 × 1,235 pixels, file size: 234 KB, MIME type: image/jpeg)
Summary
| Description |
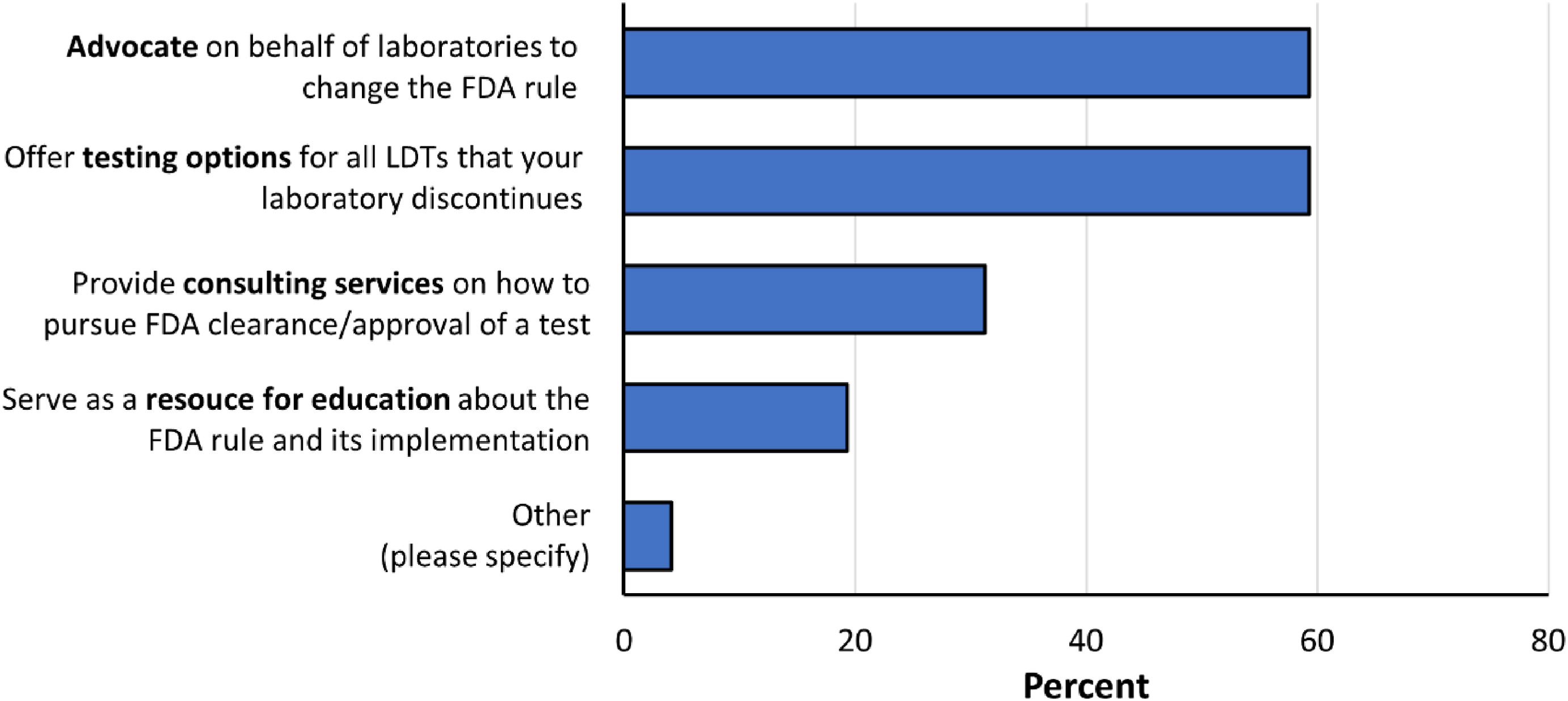
Figure 5. Expected support from reference laboratories. Respondents were asked which types of support they would need from their reference laboratories if the FDA were to adopt the proposed rule. Respondents were instructed to please select their top two types of support. |
|---|---|
| Source |
Smith, Leslie; Carricaburu, Lisa A.; Genzen, Jonathan R. (2024). "The US FDA’s proposed rule on laboratory-developed tests: Impacts on clinical laboratory testing". Practical Laboratory Medicine In press: e00407. doi:10.1016/j.plabm.2024.e00407. |
| Date |
2024 |
| Author |
Smith, Leslie; Carricaburu, Lisa A.; Genzen, Jonathan R. |
| Permission (Reusing this file) |
Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International |
| Other versions |
Licensing
|
|
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 20:20, 26 May 2024 |  | 2,764 × 1,235 (234 KB) | Shawndouglas (talk | contribs) |
You cannot overwrite this file.
File usage
The following page uses this file:









