Difference between revisions of "File:Fig2 Smith PracLabMed2024 InPress.jpg"
Shawndouglas (talk | contribs) |
Shawndouglas (talk | contribs) (Added summary.) |
||
| Line 1: | Line 1: | ||
==Summary== | |||
{{Information | |||
|Description='''Figure 2.''' Responses to survey questions. Respondents were asked ('''a''') whether they support the FDA proposal to regulate LDTs, ('''b''') whether they perform LDTs within their laboratories, ('''c''') if their laboratories would be negatively impacted by the proposed rule if enacted, ('''d''') if they anticipate having to remove tests from their laboratory menus if the proposed rule is enacted, and ('''e''') whether they have the financial resources to pay for FDA user fees. Questions for ('''c'''), ('''d'''), and ('''e''') were asked only of respondents whose laboratories performed LDTs. | |||
|Source={{cite journal |title=The US FDA’s proposed rule on laboratory-developed tests: Impacts on clinical laboratory testing |journal=Practical Laboratory Medicine |author=Smith, Leslie; Carricaburu, Lisa A.; Genzen, Jonathan R. |volume=In press |at=e00407 |year=2024 |doi=10.1016/j.plabm.2024.e00407}} | |||
|Author=Smith, Leslie; Carricaburu, Lisa A.; Genzen, Jonathan R. | |||
|Date=2024 | |||
|Permission=[http://creativecommons.org/licenses/by-nc-nd/4.0/ Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International] | |||
}} | |||
== Licensing == | == Licensing == | ||
{{cc-by-nc-nd-4.0}} | {{cc-by-nc-nd-4.0}} | ||
Latest revision as of 20:06, 26 May 2024
Summary
| Description |
Figure 2. Responses to survey questions. Respondents were asked (a) whether they support the FDA proposal to regulate LDTs, (b) whether they perform LDTs within their laboratories, (c) if their laboratories would be negatively impacted by the proposed rule if enacted, (d) if they anticipate having to remove tests from their laboratory menus if the proposed rule is enacted, and (e) whether they have the financial resources to pay for FDA user fees. Questions for (c), (d), and (e) were asked only of respondents whose laboratories performed LDTs. |
|---|---|
| Source |
Smith, Leslie; Carricaburu, Lisa A.; Genzen, Jonathan R. (2024). "The US FDA’s proposed rule on laboratory-developed tests: Impacts on clinical laboratory testing". Practical Laboratory Medicine In press: e00407. doi:10.1016/j.plabm.2024.e00407. |
| Date |
2024 |
| Author |
Smith, Leslie; Carricaburu, Lisa A.; Genzen, Jonathan R. |
| Permission (Reusing this file) |
Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International |
| Other versions |
Licensing
|
|
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 19:41, 26 May 2024 | 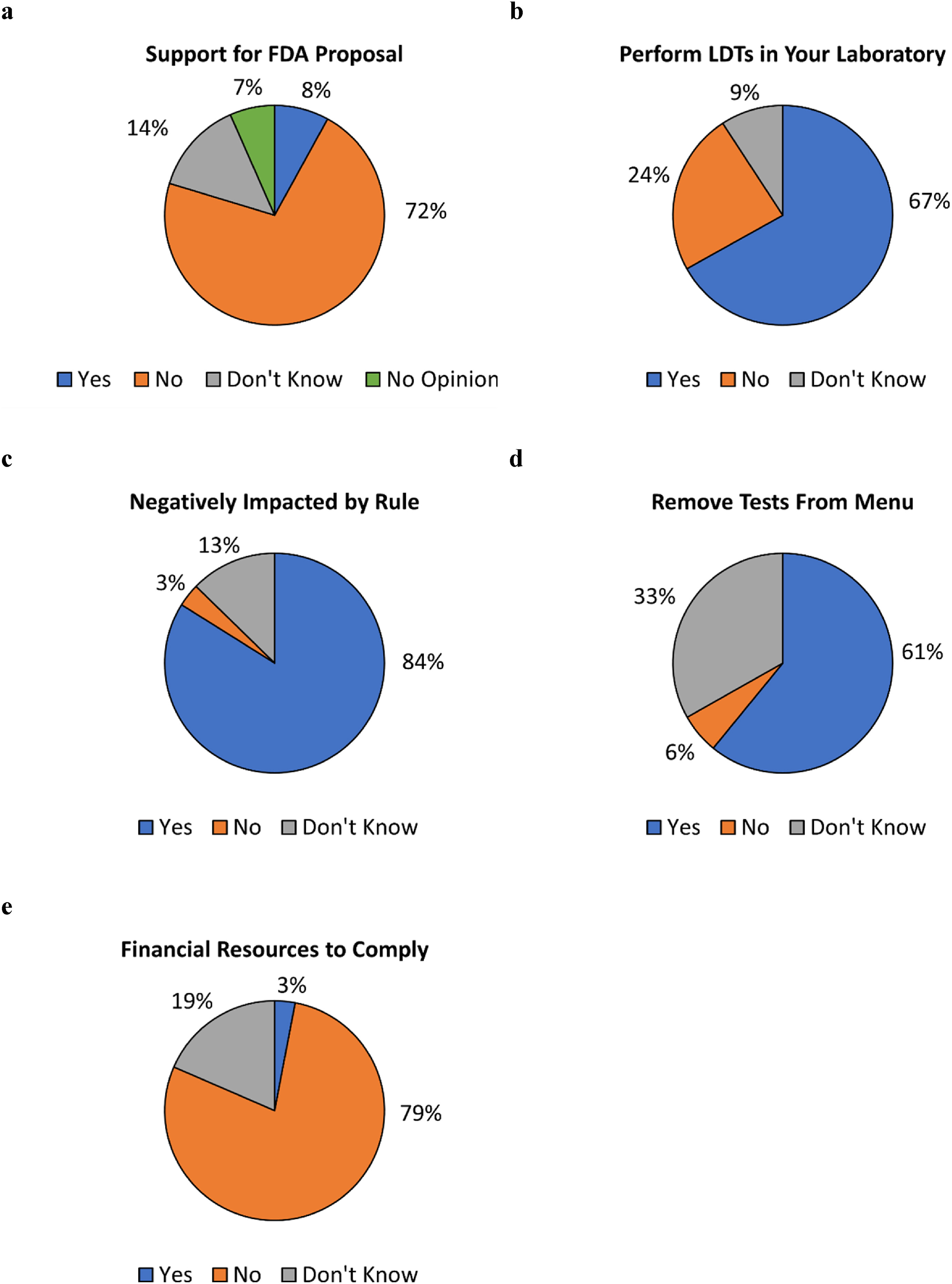 | 2,764 × 3,728 (450 KB) | Shawndouglas (talk | contribs) |
You cannot overwrite this file.
File usage
The following page uses this file:









