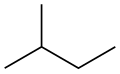
Isopentane
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylbutane[1] | |
| Other names
Isopentane
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1730723 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.039 |
| EC Number |
|
| 49318 | |
| MeSH | isopentane |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1265 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H12 | |
| Molar mass | 72.151 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Gasoline-like |
| Density | 616 mg mL−1[2] |
| Melting point | −161 to −159 °C; −258 to −254 °F; 112 to 114 K |
| Boiling point | 27.8 to 28.2 °C; 81.9 to 82.7 °F; 300.9 to 301.3 K |
| Vapor pressure | 76.992 kPa (at 20 °C) |
Henry's law
constant (kH) |
7.2 nmol Pa−1 kg−1 |
| UV-vis (λmax) | 192 nm |
Refractive index (nD)
|
1.354 |
| Viscosity | 0.214 cP (at 20 °C) |
| Thermochemistry | |
Heat capacity (C)
|
164.85 J K−1 mol−1 |
Std molar
entropy (S⦵298) |
260.41 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−179.1–−177.3 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
~ 3.3 MJ mol−1, 19,664 Btu/lb |
| Hazards | |
| GHS labelling: | |
   
| |
| Danger | |
| H224, H301, H302, H305, H336, H411 | |
| P210, P261, P273, P301+P310, P331 | |
| NFPA 704 (fire diamond) | |
| Flash point | −51 °C (−60 °F; 222 K) |
| 420 °C (788 °F; 693 K) | |
| Explosive limits | 1.4–8.3% |
| Related compounds | |
Related alkanes
|
|
Related compounds
|
2-Ethyl-1-butanol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isopentane, also called methylbutane or 2-methylbutane, is a branched-chain saturated hydrocarbon (an alkane) with five carbon atoms, with formula C
5H
12 or CH(CH
3)
2(C
2H
5).
Isopentane is a volatile and flammable liquid. It is one of three structural isomers with the molecular formula C5H12, the others being pentane (n-pentane) and neopentane (2,2-dimethylpropane).
Isopentane is commonly used in conjunction with liquid nitrogen to achieve a liquid bath temperature of −160 °C. Natural gas typically contains 1% or less isopentane,[3] but it is a significant component of natural gasoline.[4]
History
Although the mixture of pentanes was first isolated from the destructive distillation (pyrolysis) products of the boghead coal by Charles Greville Williams in 1862.[5] In 1864–1865 two chemists tried to extract same hydrocarbons from the Pennsylvanian oil. Carl Schorlemmer noted "that a mere trace of the liquid boiled below 30°C",[6] but the first to properly separate isomers (and thus discover isopentane) was American chemist Cyrus Warren (1824–1891) slightly later, who measured the boiling point of the more volatile one at 30°C.[7]
Nomenclature
The traditional name isopentane, attested in English as early as 1875,[8] was still retained in the 1993 IUPAC recommendations,[9][10] but is no longer recommended according to the 2013 recommendations.[1] The preferred IUPAC name is the systematic name 2-methylbutane. An isopentyl group is a subset of the generic pentyl group. It has the chemical structure -CH3CH2CH(CH3)2.
Uses
Isopentane is used in a closed loop in geothermal power production to drive turbines.[11]
Isopentane is used, in conjunction with dry ice or liquid nitrogen, to freeze tissues for cryosectioning in histology.
Isopentane is a major component (sometimes 30% or more) of natural gasoline, an analog of common petroleum-derived gasoline that is condensed from natural gas.[4] Its share in commercial car fuel is highly variable: 19–45% in 1990s Sweden,[13] 4–31% in 1990s US[14] and 3.6–11% in the US in 2011.[15] It has a substantially higher octane rating (RON 93.7) than n-pentane (61.7), and therefore there is interest in conversion from the latter.[16]
References
- ^ a b Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 652. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The names 'isobutane', 'isopentane' and 'neopentane' are no longer recommended.
- ^ James Wei (1999), Molecular Symmetry, Rotational Entropy, and Elevated Melting Points. Ind. Eng. Chem. Res., volume 38 issue 12, pp. 5019–5027 doi:10.1021/ie990588m
- ^ Georg Hammer, Torsten Lübcke, Roland Kettner, Mark R. Pillarella, Herta Recknagel, Axel Commichau, Hans-Joachim Neumann and Barbara Paczynska-Lahme "Natural Gas" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a17_073.pub2
- ^ a b Ivan F. Avery, L. V. Harvey (1958): Natural-gasoline and Cycling Plants in the United States, Information circular, U.S. Department of the Interior, Bureau of Mines. 12 pages.
- ^ Williams, C. Greville (1862). "On the hydrocarbons produced by destructive distillation of boghead coal" (PDF). Journal of the Chemical Society. 15: 130–134. doi:10.1039/JS8621500130. ISSN 0368-1769.
- ^ Proceedings of the Literary and Philosophical Society of Manchester. 1864.
- ^ The American Journal of Science. Kline Geology Laboratory, Yale University. 1865.
- ^ Watts (F.C.S.), Henry (1875). A Dictionary of Chemistry and Allied Branches of Other Sciences.
- ^ Table 19(a) Acyclic and monocyclic hydrocarbons. Parent hydrocarbons
- ^ Panico, R. & Powell, W. H., eds. (1994). A Guide to IUPAC Nomenclature of Organic Compounds 1993. Oxford: Blackwell Science. ISBN 0-632-03488-2.
- ^ Byproduct Isopentane also used in some of the LPG plant to run the boiler and generate the power. HS Orka HF Energy Plant IV Archived 2014-10-18 at the Wayback Machine
- ^ "Animal Resources Program - the Office of the Vice President for Research | UAB".
- ^ Östermark, Ulf; Petersson, Göran (1992-09-01). "Assessment of hydrocarbons in vapours of conventional and alkylate-based petrol" (PDF). Chemosphere. 25 (6): 763–768. Bibcode:1992Chmsp..25..763O. doi:10.1016/0045-6535(92)90066-Z. ISSN 0045-6535.
- ^ Doskey, Paul V.; Porter, Joseph A.; Scheff, Peter A. (November 1992). "Source Fingerprints for Volatile Non-Methane Hydrocarbons". Journal of the Air & Waste Management Association. 42 (11): 1437–1445. Bibcode:1992JAWMA..42.1437D. doi:10.1080/10473289.1992.10467090. ISSN 1047-3289.
- ^ "Hydrocarbon Composition of Gasoline Vapor Emissions from Enclosed Fuel Tanks". nepis.epa.gov. United States Environmental Protection Agency. 2011.
- ^ Sheng Wang, Ying Zhang, Mao-Gang He, Xiong Zheng, and Li-Bin Chen (2014): "Thermal Diffusivity and Speed of Sound of Saturated Pentane from Light Scattering". International Journal of Thermophysics, volume 35, pages 1450–1464. doi:10.1007/s10765-014-1718-x
External links
- International Chemical Safety Card 1153
- IUPAC Nomenclature of Organic Chemistry (online version of the "Blue Book")
Notes
This article is a direct transclusion of the Wikipedia article and therefore may not meet the same editing standards as LIMSwiki.










