Journal:Leaner and greener analysis of cannabinoids
| Full article title | Leaner and greener analysis of cannabinoids |
|---|---|
| Journal | Analytical and Bioanalytical Chemistry |
| Author(s) | Mudge, Elizabeth M.; Murch, Susan J.; Brown, Paula N. |
| Author affiliation(s) | British Columbia Institute of Technology, University of British Columbia |
| Primary contact | Email: Paula underscore brown at bcit dot ca |
| Year published | 2017 |
| Volume and issue | 409(12) |
| Page(s) | 3153–63 |
| DOI | 10.1007/s00216-017-0256-3 |
| ISSN | 1618-2650 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://link.springer.com/article/10.1007%2Fs00216-017-0256-3 |
| Download | https://link.springer.com/content/pdf/10.1007%2Fs00216-017-0256-3.pdf (PDF) |
|
|
This article should not be considered complete until this message box has been removed. This is a work in progress. |
Abstract
There is an explosion in the number of labs analyzing cannabinoids in marijuana (Cannabis sativa L., Cannabaceae); however, existing methods are inefficient, require expert analysts, and use large volumes of potentially environmentally damaging solvents. The objective of this work was to develop and validate an accurate method for analyzing cannabinoids in cannabis raw materials and finished products that is more efficient and uses fewer toxic solvents. A method using high-performance liquid chromatography (HPLC) with diode-array detection (DAD) was developed for eight cannabinoids in Cannabis flowers and oils using a statistically guided optimization plan based on the principles of green chemistry. A single-laboratory validation determined the linearity, selectivity, accuracy, repeatability, intermediate precision, limit of detection, and limit of quantitation of the method. Amounts of individual cannabinoids above the limit of quantitation in the flowers ranged from 0.02 to 14.9% concentration (w/w), with repeatability ranging from 0.78 to 10.08% relative standard deviation. The intermediate precision determined using Horwitz ratios (HorRat) ranged from 0.3 to 2.0. The limits of quantitation (LoQs) for individual cannabinoids in flowers ranged from 0.02 to 0.17% w/w. This is a significant improvement over previous methods and is suitable for a wide range of applications, including regulatory compliance, clinical studies, direct patient medical services, and commercial suppliers.
Keywords: green chemistry, single-laboratory validation, Cannabis, cannabinoids, medical marijuana
Introduction
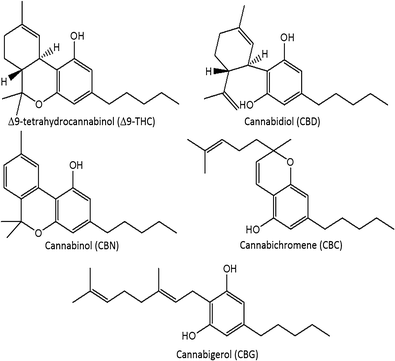
The modern cannabis market is in a period of dramatic flux. In the United States, cannabis is classified as a Schedule I drug[1]; however, eight U.S. states have legalized marijuana for recreational use, and 28 states have allowed medical marijuana on the basis of evidence of anxiolytic, analgesic, sedative, anticancer, and appetite stimulation effects.[2][3][4][5] Regulations regarding Cannabis spp. vary globally. The Netherlands, Uruguay, and Portugal have decriminalized possession. In Canada, cannabis is a Schedule II controlled substance, but regulations have allowed production for medical purposes through licensed producers and personal production licenses.[6] Canadian production of commercial products must take place in a facility using good manufacturing practices, and products must be assayed for the presence and quantity of Δ9-tetrahydrocannabinol (Δ9-THC), Δ9-tetrahydrocannabinolic acid (THCA), cannabidiol (CBD), and cannabidiolic acid (CBDA), using validated analytical methods.[6] In total, more than 100 cannabinoids in 11 subclasses have been characterized in cannabis and are concentrated in the glandular trichomes of the female inflorescences. Other cannabinoid classes include cannabigerol (CBG), cannabichromene (CBC), and cannabinol (CBN) (Fig. 1).[7] The cannabinoids occur primarily in acid form, with neutral cannabinoids formed during drying, storage, and decarboxylation during smoking. Δ9-THC, the main psychoactive cannabinoid, can be over 20% by weight in specially bred cannabis strains.[8][9] CBD, known for its anti-inflammatory activity and antagonism of Δ9-THC-induced anxiety, can range from below 0.5% up to 6.5% by weight.[9][10]
|
There are a significant number of analytical methods to quantify cannabinoids available, many of which do not provide sufficient validation data to establish the method performance and reliability. Without this information, there is a possibility that the methods are not fit for purpose. The solvent composition, mass-to-solvent ratio, extraction technique, and time vary considerably between methods. Separations of cannabinoids use different mobile phases, columns, and gradients, and given the number of minor cannabinoids present in authentic materials, there is a possibility for coelution of peaks and inaccurate quantitative results.[11][12] Rigorous validation procedures are necessary to ensure that the results of any analytical method are reliable. Without this data on method performance, the final method may not meet the needs of the users who adopt it for routine use, therefore producing inaccurate information pertaining to the products that people are using for the treatment of medical conditions.[13] The speed with which regulations have changed and the nature of the rapidly expanding cannabis marketplace have created increased pressure for fast, safe, simple, and accurate analysis of phytochemicals to meet the demands of high-throughput laboratories and rapid release of finished products.
The most commonly used extraction solvent for cannabinoid analysis is 9:1 methanol/chloroform (% v/v), with some exceptions.[9][11][14][15][16] It was originally selected to dissolve the internal standard di-n-octyl phthalate, which is no longer necessary with commercially available reference standards.[16] There is an increasing desire to find greener methods to reduce use of chlorinated solvents, which can be toxic, expensive to dispose of, and hazardous to transport and store.[17][18] Long-term, chronic exposure to chloroform is associated with liver and kidney damage, where the occupational exposure limit is 2 ppm in air.[18][19] While laboratory safety procedures reduce exposure significantly, the risks of spills and inhalation of vapors are increased with chloroform use, and there is a diversity of safety equipment used in the labs engaged in this analysis. Removal of chloroform from the extraction solvent will improve laboratory safety and reduce reagent and disposal costs, while improving the environmental impact associated with chlorinated solvent usage.
The objective of the current work was to develop a fully validated, simplified, green chemistry method for labs to implement that may not have high levels of expertise or capacity for method development or validation. We developed the method using statistically guided method development protocols for the quantitation of eight cannabinoids in Cannabis flowers and oils. Nine authentic Cannabis flower materials and one cannabis oil with a wide range of cannabinoid contents were obtained and used as test articles for the validation of the method of the AOAC International guidelines.[20] This method does not use chlorinated solvents, reduces sample preparation time, and ensures precise and accurate determination of cannabinoids.
Materials and methods
Reagents
Methanol and acetonitrile suitable for high-performance liquid chromatography (HPLC) were purchased from VWR International (Mississauga, ON, Canada). Chloroform suitable for the American Chemical Society (ACS) was obtained from VWR International. Water was purified to 18 MΩ using a Barnstead Smart2Pure nanopure system (Thermo Scientific, Waltham, MA). Ammonium formate for HPLC (>99.0%) was purchased from Sigma Aldrich (Oakville, ON, Canada), and formic acid (98% pure) was purchased from Fisher Scientific (Ottawa, ON, Canada).
Calibration standards
Certified reference materials (CRMs) were purchased from Cerilliant Corp. (Round Rock, TX) for nine cannabinoids: Δ9-THC, THCA, Δ8-THC, CBD, CBDA, CBG, CBN, CBC, and tetrahydrocannabivarin (THCV). The individual cannabinoids were provided in solution at 1.0 mg/mL concentration, certified by the supplier. The acidic cannabinoids were provided in acetonitrile and neutral cannabinoids in methanol. Fresh ampules were used for the validation study to ensure accurate quantitation of the individual constituents.
Test materials
Dried medical marijuana samples were purchased from several licensed producers within Canada. Nine products were selected for a variety of cannabinoid concentrations ranging from 0.2% to 17% total THC and 0.3% to 9% total CBD. As a result of the legal restrictions pertaining to these products, voucher specimens were not possible, but the samples were purchased directly from the source to ensure authenticity. A dried ethanol extract was dissolved in oil at a 1:10 dilution.
HPLC analysis
An Agilent 1200 RRLC system equipped with a temperature-controlled autosampler, binary pump, and diode-array detector (Agilent Technologies, Mississauga, ON, Canada) was used to separate the cannabinoids. The separation was achieved on a Kinetex C18, 1.7 μm, 100 × 3.0 mm i.d. column (Phenomenex, Torrance, CA). Mobile phase compositions were (A) 10 mM ammonium formate, pH 3.6 and (B) acetonitrile using gradient conditions at 0.6 mL/min. The separation was achieved according to the following gradient: 0–8 min, 52–66%B; 8–8.5 min, 66–70%B; 8.5–13 min, 70–80%B; 13–15 min, 80%B. A 7-min column equilibration was performed after each run. The injection volume was 5 μL and detection was at 220 nm. The autosampler was maintained at 4 °C.
Preparation of test materials
Plant tissues
A minimum of five grams of dried flowers was ground together from each test sample to ensure sample homogeneity. Ground flowers were extracted by weighing 200.0 mg into a 50-mL amber centrifuge tube. Then 25.00 mL of 80% methanol was added and vortexed for 30 seconds. Extraction took place using a sonicating bath for 15 minutes, where samples were vortexed every five minutes. Extracts were filtered with a 0.22-μm Teflon filter; diluted at 1:2, 1:5, or 1:10 using the extraction solvent into amber HPLC vials; and stored at 4 °C until analysis.
Oil
Cannabis oil was mixed by inversion prior to sample preparation. Then 50.0 mg of oil was weighed into a 50-mL amber centrifuge tube, to which 10.00 mL of methanol was added and vortexed for 30 seconds. Extracts were sonicated for 15 minutes, with vortexing every five minutes. Samples were filtered with 0.22-μm Teflon filters into amber HPLC vials and stored at 4 °C until analysis.
Method optimization
Analyte stability
Mixed calibration standards were stored at −20 °C, 4 °C, and 22 °C in the dark and tested at regular intervals to assess cannabinoid stability in solutions. Sample extracts were stored at 4 °C and 22 °C in light and dark conditions. A sample with greater than 5% loss from time zero was considered unstable.
Fractional factorial
The partial factorial design for method optimization and data analysis was completed using Minitab 16 (Minitab 16, State College, PA). Individual cannabinoids were quantified as percentage weight for weight in Cannabis flowers and milligrams per gram in oil. Microsoft Excel (Richmond, WA) was used for quantitative calculations and statistical analysis of validation data.
Single-laboratory validation parameters
The optimized method was subjected to a single-laboratory validation according to AOAC International guidelines for dietary supplements.[20] Δ8-THC was not observed in any of the samples and therefore was not considered in the method validation.
Preparation of calibration solutions
Individual cannabinoid CRMs were used to prepare seven-point standard calibration curves for eight cannabinoids in concentrations ranging from 0.5 to 250 μg/mL. Dilutions of the CRMs were performed using the extraction solvent composed of 80% methanol. Concentration ranges were modified for each cannabinoid as summarized in Table 1. The calibration curves were plotted, and the slope and y intercept for each cannabinoid were used for linear regression analysis. Calibration curves were visually inspected and correlation coefficients were determined. An r2 of at least 0.995 was deemed suitable for quantitation. Mixed standards were stored at 4 °C and were stable for up to three days.
| |||||||||||||||||||||||||||||||||||||
Acknowledgements
Author contributions
Funding
Conflicts of interest
References
- ↑ Lamarine, R.J. (2012). "Marijuana: Modern medical chimaera". Journal of Drug Education 42 (1): 1–11. doi:10.2190/DE.42.1.a. PMID 22873011.
- ↑ Porter, B.E.; Jacobson, C. (2013). "Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy". Epilepsy & Behavior 29 (3): 574–7. doi:10.1016/j.yebeh.2013.08.037. PMC PMC4157067. PMID 24237632. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4157067.
- ↑ Tafelski, S.; Häuser, W.; Schäfer, M. (2016). "Efficacy, tolerability, and safety of cannabinoids for chemotherapy-induced nausea and vomiting--a systematic review of systematic reviews". Schmerz 30 (1): 14–24. doi:10.1007/s00482-015-0092-3. PMID 26787227.
- ↑ Whiting, P.F.; Wolff, R.F.; Deshpande, S. et al. (2015). "Cannabinoids for Medical Use: A Systematic Review and Meta-analysis". JAMA 313 (24): 2456–73. doi:10.1001/jama.2015.6358. PMID 26103030.
- ↑ Bagshaw, S.M.; Hagen, N.A. (2002). "Medical efficacy of cannabinoids and marijuana: A comprehensive review of the literature". Journal of Palliative Care 18 (2): 111–22. doi:10.1177/082585970201800207. PMID 12164099.
- ↑ 6.0 6.1 Government of Canada (2016). "Access to Cannabis for Medical Purposes Regulations (SOR/2016-230)". Justice Laws Website. https://laws.justice.gc.ca/eng/regulations/sor-2016-230/.
- ↑ Mahlberg, P.G.; Kim, E.S. (2004). "Accumulation of Cannabinoids in Glandular Trichomes of Cannabis (Cannabaceae)". Journal of Industrial Hemp 9 (1): 15–36. doi:10.1300/J237v09n01_04.
- ↑ Brenneisen, R. (2007). "Chapter 2: Chemistry and Analysis of Phytocannabinoids and Other Cannabis Constituents". In ElSohly, M.A.. Marijuana and the Cannabinoids. Humana Press. pp. 17–49. doi:10.1007/978-1-59259-947-9. ISBN 9781592599479.
- ↑ 9.0 9.1 9.2 Swift, W.; Wong, A.; Li, K.M. et al. (2013). "Analysis of cannabis seizures in NSW, Australia: Cannabis potency and cannabinoid profile". PLoS One 8 (7): e70052. doi:10.1371/journal.pone.0070052. PMC PMC3722200. PMID 23894589. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3722200.
- ↑ Zuardi, A.W.; Shirakawa, I.; Finkelfarb, E. et al. (1982). "Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects". Psychopharmacology 76 (3): 245–50. PMID 6285406.
- ↑ 11.0 11.1 Lehmann, T.; Brenneisen, R. (1995). "High Performance Liquid Chromatographic Profiling of Cannabis Products". Journal of Liquid Chromatography 18 (4): 689–700. doi:10.1080/10826079508009265.
- ↑ Gul, W.; Gul, S.W.; Radwan, M.M. et al. (2015). "Determination of 11 Cannabinoids in Biomass and Extracts of Different Varieties of Cannabis Using High-Performance Liquid Chromatography". Journal of AOAC International 98 (6): 1523–8. doi:10.5740/jaoacint.15-095. PMID 26651563.
- ↑ Mudge, E.M.; Betz, J.M.; Brown, P.N. (2016). "The Importance of Method Selection in Determining Product Integrity for Nutrition Research". Advances in Nutrition 7 (2): 390-8. doi:10.3945/an.115.010611. PMC PMC4785475. PMID 26980823. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4785475.
- ↑ De Backer, B.; Debrus, B.; Lebrun, P. et al. (2009). "Innovative development and validation of an HPLC/DAD method for the qualitative and quantitative determination of major cannabinoids in cannabis plant material". Journal of Chromatography B 877 (32): 4115-24. doi:10.1016/j.jchromb.2009.11.004. PMID 19932642.
- ↑ Mehmedic, Z.; Chandra, S.; Slade, D. et al. (2010). "Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008". Journal of Forensic Sciences 55 (5): 1209–17. doi:10.1111/j.1556-4029.2010.01441.x. PMID 20487147.
- ↑ 16.0 16.1 Smith, R.N.; Vaughan, C.G. (1976). "High-pressure liquid chromatography of cannabis. Quantitative analysis of acidic and neutral cannabinoids". Journal of Chromatography 129: 347–54. doi:10.1016/s0021-9673(00)87794-8. PMID 12189.
- ↑ Alfonsi, K.; Colberg, J.; Dunn, P.J. et al. (2008). "Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation". Green Chemistry 10 (1): 31–36. doi:10.1039/B711717E.
- ↑ 18.0 18.1 Watts, P.; Long, G.; Meek, M.E. (2004). "Chloroform" (PDF). Concise International Chemical Assessment Document 58. World Health Organization. https://www.who.int/ipcs/publications/cicad/en/cicad58.pdf.
- ↑ National Institute for Occupational Safety and Health (September 2007). "Chloroform". NIOSH Pocket Guide to Chemical Hazards. Centers for Disease Control and Prevention. https://www.cdc.gov/niosh/npg/npgd0127.html. Retrieved 24 October 2016.
- ↑ 20.0 20.1 "Appendix K, Part I: AOAC guidelines for single-laboratory validation of chemical methods for dietary supplements and botanicals". Official Methods of Analysis of AOAC International. 2013. pp. 1–32.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added.