Difference between revisions of "Template:Article of the week"
Shawndouglas (talk | contribs) (Updated article of the week text.) |
Shawndouglas (talk | contribs) (Updated article of the week text) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File: | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Arendt ClinEpidem2020 12.jpg|240px]]</div> | ||
'''"[[Journal: | '''"[[Journal:Existing data sources in clinical epidemiology: Laboratory information system databases in Denmark|Existing data sources in clinical epidemiology: Laboratory information system databases in Denmark]]"''' | ||
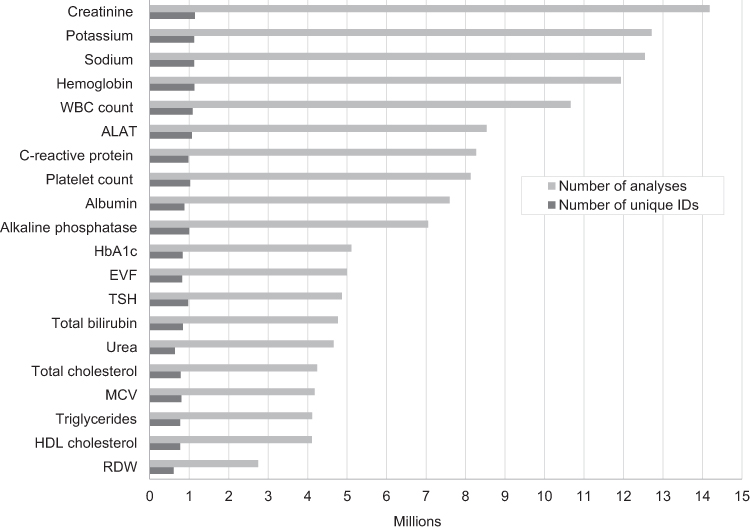
Routine [[biomarker]] results from [[hospital]] [[laboratory information system]]s (LIS)—covering hospitals and general practitioners—in Denmark are available to researchers through access to the regional Clinical Laboratory Information System Research Database at Aarhus University and the nationwide Register of Laboratory Results for Research. This review describes these two data sources. The [[laboratory]] databases have different geographical and temporal coverage. They both include individual-level biomarker results that are electronically transferred from LISs. The biomarker results can be linked to all other Danish registries at the individual level using the unique identifier, the CPR number. ('''[[Journal:Existing data sources in clinical epidemiology: Laboratory information system databases in Denmark|Full article...]]''')<br /> | |||
<br /> | <br /> | ||
''Recently featured'': | ''Recently featured'': | ||
: ▪ [[Journal:HEnRY: A DZIF LIMS tool for the collection and documentation of biospecimens in multicentre studies|HEnRY: A DZIF LIMS tool for the collection and documentation of biospecimens in multicentre studies]] | |||
: ▪ [[Journal:Bringing big data to bear in environmental public health: Challenges and recommendations|Bringing big data to bear in environmental public health: Challenges and recommendations]] | : ▪ [[Journal:Bringing big data to bear in environmental public health: Challenges and recommendations|Bringing big data to bear in environmental public health: Challenges and recommendations]] | ||
: ▪ [[Journal:Enzyme immunoassay for measuring aflatoxin B1 in legal cannabis|Enzyme immunoassay for measuring aflatoxin B1 in legal cannabis]] | : ▪ [[Journal:Enzyme immunoassay for measuring aflatoxin B1 in legal cannabis|Enzyme immunoassay for measuring aflatoxin B1 in legal cannabis]] | ||
Revision as of 22:01, 9 August 2021
"Existing data sources in clinical epidemiology: Laboratory information system databases in Denmark"
Routine biomarker results from hospital laboratory information systems (LIS)—covering hospitals and general practitioners—in Denmark are available to researchers through access to the regional Clinical Laboratory Information System Research Database at Aarhus University and the nationwide Register of Laboratory Results for Research. This review describes these two data sources. The laboratory databases have different geographical and temporal coverage. They both include individual-level biomarker results that are electronically transferred from LISs. The biomarker results can be linked to all other Danish registries at the individual level using the unique identifier, the CPR number. (Full article...)
Recently featured: