Difference between revisions of "Template:Article of the week"
Shawndouglas (talk | contribs) (Updated article of the week text) |
Shawndouglas (talk | contribs) (Updated article of the week text) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File: | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:GA Hauschild iScience2022 25-12.jpg|240px]]</div> | ||
'''"[[Journal: | '''"[[Journal:Guideline for software life cycle in health informatics|Guideline for software life cycle in health informatics]]"''' | ||
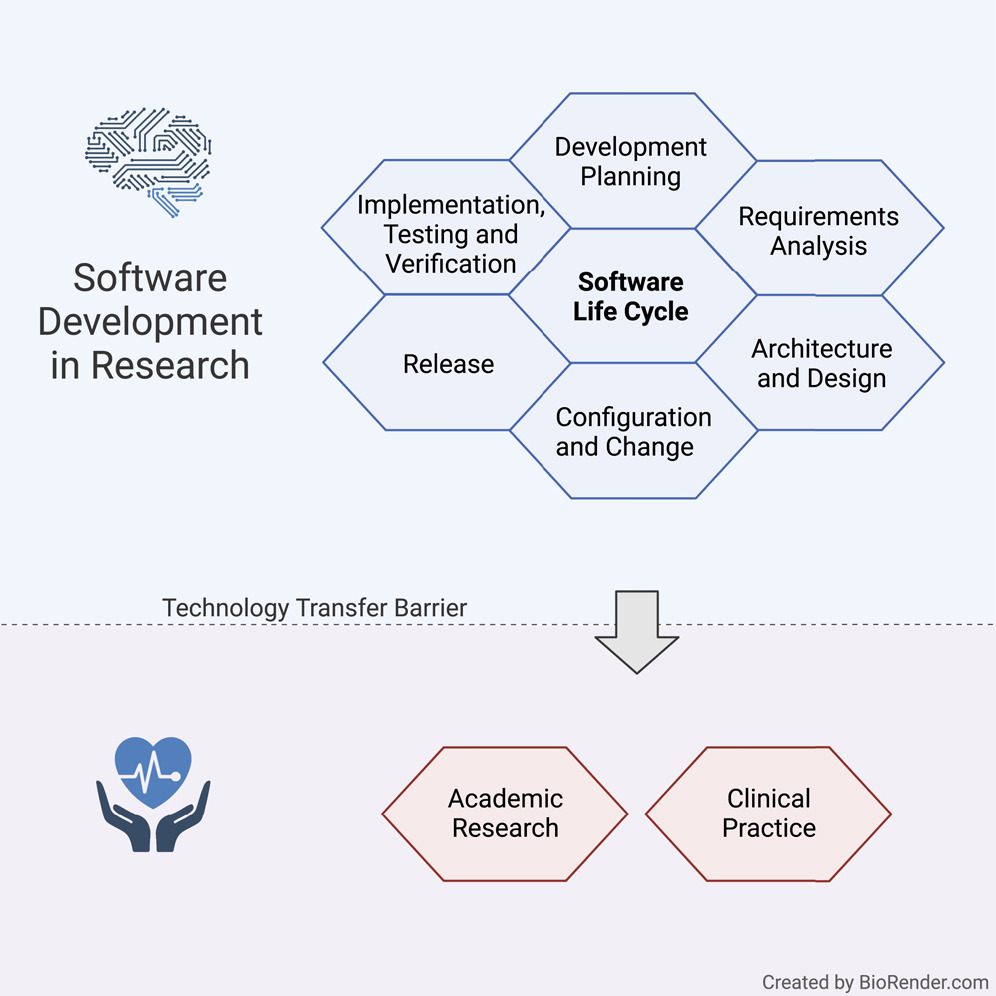
The | The long-lasting trend of [[medical informatics]] is to adapt novel technologies in the medical context. In particular, incorporating [[artificial intelligence]] (AI) to support clinical decision-making can significantly improve monitoring, diagnostics, and prognostics for the patient’s and medic’s sake. However, obstacles hinder a timely technology transfer from the medical research setting to the actual clinical setting. Due to the pressure for novelty in the [[research]] context, projects rarely implement [[Quality (business)|quality]] standards. Here, we propose a guideline for academic software life cycle (SLC) processes tailored to the needs and capabilities of research organizations ... ('''[[Journal:Guideline for software life cycle in health informatics|Full article...]]''')<br /> | ||
''Recently featured'': | ''Recently featured'': | ||
{{flowlist | | {{flowlist | | ||
* [[Journal:Development of an integrated and comprehensive clinical trial process management system|Development of an integrated and comprehensive clinical trial process management system]] | |||
* [[Journal:A web application to support the coordination of reflexive, interpretative toxicology testing|A web application to support the coordination of reflexive, interpretative toxicology testing]] | * [[Journal:A web application to support the coordination of reflexive, interpretative toxicology testing|A web application to support the coordination of reflexive, interpretative toxicology testing]] | ||
* [[Journal:ApE, A Plasmid Editor: A freely available DNA manipulation and visualization program|ApE, A Plasmid Editor: A freely available DNA manipulation and visualization program]] | * [[Journal:ApE, A Plasmid Editor: A freely available DNA manipulation and visualization program|ApE, A Plasmid Editor: A freely available DNA manipulation and visualization program]] | ||
}} | }} | ||
Revision as of 15:18, 23 October 2023
"Guideline for software life cycle in health informatics"
The long-lasting trend of medical informatics is to adapt novel technologies in the medical context. In particular, incorporating artificial intelligence (AI) to support clinical decision-making can significantly improve monitoring, diagnostics, and prognostics for the patient’s and medic’s sake. However, obstacles hinder a timely technology transfer from the medical research setting to the actual clinical setting. Due to the pressure for novelty in the research context, projects rarely implement quality standards. Here, we propose a guideline for academic software life cycle (SLC) processes tailored to the needs and capabilities of research organizations ... (Full article...)
Recently featured: