Journal:Development of an integrated and comprehensive clinical trial process management system
| Full article title | Development of an integrated and comprehensive clinical trial process management system |
|---|---|
| Journal | BMC Medical Informatics and Decision Making |
| Author(s) | Shen, Liang; Zhai, You; Pan, AXiang; Zhao, Qingwei; Zhou, Min; Lio, Jian |
| Author affiliation(s) | Zhejiang University School of Medicine |
| Primary contact | Email: minzhou at zju dot edu dot cn |
| Year published | 2023 |
| Volume and issue | 23 |
| Article # | 61 |
| DOI | 10.1186/s12911-023-02158-8 |
| ISSN | 1472-6947 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-023-02158-8 |
| Download | https://bmcmedinformdecismak.biomedcentral.com/counter/pdf/10.1186/s12911-023-02158-8.pdf (PDF) |
Abstract
Background: The process of initiating and completing clinical drug trials in hospital settings is highly complex, with numerous institutional, technical, and record-keeping barriers. In this study, we independently developed an integrated clinical trial management system (CTMS) designed to comprehensively optimize the process management of clinical trials. The CTMS includes system development methods, efficient integration with external business systems, terminology, and standardization protocols, as well as data security and privacy protection.
Methods: The development process proceeded through four stages, including demand analysis and problem collection, system design, system development and testing, system trial operation, and training the whole hospital to operate the system. The integrated CTMS comprises three modules: project approval and review management, clinical trial operations management, and background management modules. These are divided into seven subsystems and 59 internal processes, realizing all the functions necessary to comprehensively perform the process management of clinical trials. Efficient data integration is realized through extract-transform-load (ETL), message queue, and remote procedure call services with external systems such as the hospital information system (HIS), laboratory information system (LIS), electronic medical record (EMR), and clinical data repository (CDR). Data security is ensured by adopting corresponding policies for data storage and data access. Privacy protection complies with laws and regulations and de-identifies sensitive patient information.
Results: The integrated CTMS was successfully developed in September 2015 and updated to version 4.2.5 in March 2021. During this period, 1,388 study projects were accepted, 43,051 electronic data files stored, and 12,144 subjects recruited in the First Affiliated Hospital, Zhejiang University School of Medicine.
Conclusion: The developed integrated CTMS realizes the data management of the entire clinical trials process, providing basic conditions for the efficient, high-quality, and standardized operation of clinical trials.
Keywords: clinical trial, clinical trial management system (CTMS), information technology, integrated management system, medical informatics
Background
Clinical trials of drugs are an important stage in drug research and development and are means to improve the development of medical science and technology.[1][2][3][4][5] Standardizing the management of clinical research is key to simplifying the research process, improving quality, and ensuring the accuracy, reliability, and integrity of the results, thereby shortening the development cycle of new drugs and accelerating the process of drug registration.[6]
In China, each hospital has a dedicated clinical trial management department, called a clinical trial institution (CTI), responsible for the administrative management of clinical trial operation in the hospital. This involves determining the project leader, formulating research plans with sponsors, reviewing the plans with the ethics committee, signing research contracts, screening and enrolling subjects, originating records of trials, receiving drugs, distributing and recovering, processing or reporting adverse events (AE) or serious adverse events (SAE), managing in-hospital quality, accepting supervision and inspection, summarizing, and filing. The traditional management mode is based on paper documents and involves considerable manual labor, which often leads to untimely information updates, ineffective management, and unverifiable quality.
Today, the rapid development of internet technology has profoundly impacted the mode of clinical trial management. Diverse companies have developed a series of network management systems for clinical trials.[7][8] Most of these systems have been developed based on the research and development (R&D) requirements of pharmaceutical companies. Clinical trials are managed in a networked manner, which only handles project-related data and excludes the process management links of the clinical trial organization. These commercialized clinical trial management systems (CTMSs) provide comprehensive clinical trial management services, supporting all types of clinical trials, from Phase I to Phase III, from a trial conducted in a single research center to multinational clinical trials; thus, they improve the quality of clinical trials and the efficiency of data management. However, from the perspective of drug clinical trial institutions, this type of CTMS cannot satisfy the practical requirements for real-time and effective management of all clinical trials carried out by these institutions. Hence, a new CTMS has been developed as an institutional management model in China. Its development and application are still in their infancy. The CTMS also faces certain incompatibility issues with the hospital database. It is difficult to achieve data sharing and docking. Moreover, the management method is still relatively primitive. The degree of digitalization, networking, and standardization of clinical trials is relatively low, and it is practically impossible for institutions to effectively manage clinical trials.
In recent years, owing to the increase in clinical trial projects in our hospital, professional and standardized information systems were urgently needed to assist in the management of clinical trials throughout the hospital. At present, the need for a centralized clinical trial project management platform reliant on the clinical data system of the hospital itself is intensifying. Based on the local area network (LAN) security architecture of our hospital, we have constructed a CTMS, which organically integrates the clinical trial organization management office, ethics committee, clinical trial center pharmacy, and clinical professional departments. The management system covers the entire process of clinical trials, including trial project establishment, ethical review, signing of agreements, trial implementation, trial conclusion, sponsor management, subject management, follow-up management, and centralized management of pharmacies supporting medication trials. The personnel involved include clinical departments, institutional management offices, central pharmacies, and ethics committees. With the help of the information system, a large number of personnel and complicated work processes are now more organically integrated to realize information sharing and collaborative work. With the help of this CTMS, the implementation of clinical trials can be standardized and the entire process can be traceable.
As a site of clinical trials, hospital management involves multi-party collaboration, as well as study project management, subject management, investigational product management, quality control, financial management, and other complex business processes. Hospitals must develop dedicated systems to assist the entire process management of clinical trials to ensure their efficient and high-quality operation.
The main objectives of this study are as follows. We aim (1) to develop an integrated CTMS as a dedicated database for clinical trials; (2) to achieve efficient data integration of the CTMS with hospital business systems such as the hospital information system (HIS), laboratory information system (LIS), clinical data repository (CDR), picture archiving and communication system (PACS), and electronic medical record (EMR); (3) to facilitate standardization and consistency of terminology in the development of database models and business processes; and (4) to ensure the safety and security of clinical data as well as protect patient privacy to comply with relevant regulations.
Methods
Overview
The First Affiliated Hospital, Zhejiang University School of Medicine (FAHZU) has six campuses with approximately 5,000 beds. In 2020, the institution recorded over 4.2 million outpatient and emergency visits, and 236,100 discharges. As one of the pioneering and earliest-founded National Drug CTIs in China, the first batch of clinical pharmacology bases under the Ministry of Health was established in 1998. As a first-class integrated service platform for clinical research in China, it operates 24 specialized groups and depends on an internationally recognized and independent ethics examination system, and it has undertaken more than 2,000 instances of foreign and domestic clinical trials since its establishment, including both Phase I–IV drugs and medical devices.
System development process
The development of an integrated CTMS began in March 2014, and after 18 months, the first version of the CTMS was launched in FAHZU in September 2015. The complete system development process was carried out in four stages, including two months of requirement analysis and problem collection, four months of system design, six months of system development and testing, and six months of system operation training and pilot runs. Based on the feedback from the use of the first version of CTMS and the continuous in-depth exploration of the business, the system’s functionality and user experience continue to be iteratively updated. By March 2021, the updated version 4.2.5 was launched. Through the construction of an integrated CTMS, the entire process of clinical trial data management is realized, which provides the basic conditions for the efficient, high-quality, and standardized operation of clinical trials in the hospital.
First stage
The collection and analysis of CTMS requirements defined the existing problem set to be solved. This stage is the most critical and determines the final goal direction of the system. The collection of requirements started in March 2014, and the participants included the CTI office director, CTI office secretary, ethics committee (EC) members, EC secretary, principal investigator (PI), sub-investigator, study nurse, clinical research associate (CRA), clinical research coordinator (CRC), investigational product custodians, financial officer, quality control expert, statisticians, data manager, pharmacovigilance associate, and information technology expert. After two months of formal and informal interviews, as well as an analysis of the collection requirements, the design goal of CTMS was determined. The overall goal is for the CTMS to realize the entire process of clinical trial management, including study project approval, ethical review, subject recruitment, subject management, investigational product management, financial management, and quality management. Table 1 lists the functional requirements for realizing the entire process management of clinical trials in hospitals based on seven dimensions. These requirements were used to construct the integrated CTMS.
| |||||||||||||||||||||||||||
Second stage
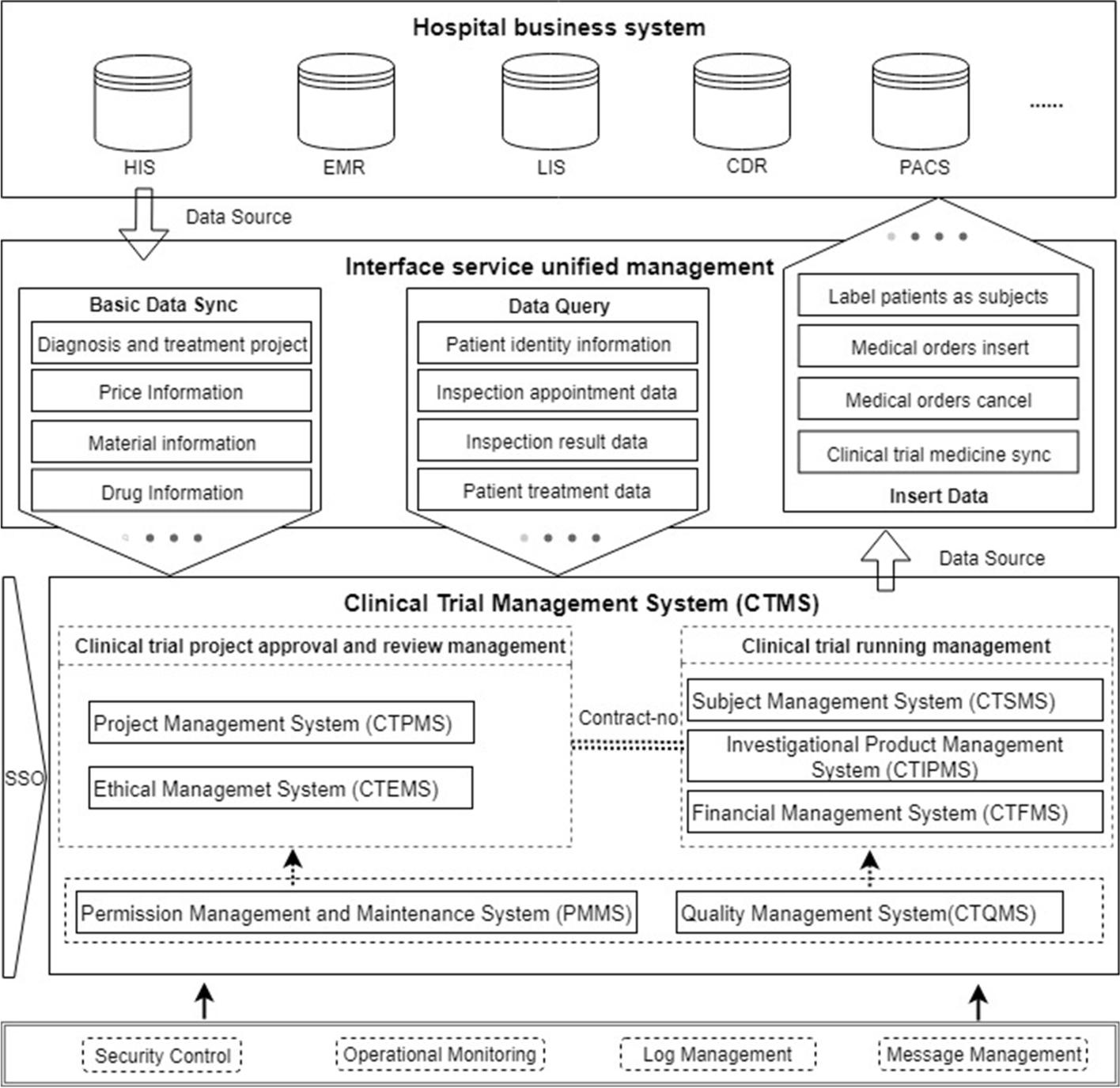
In the second stage, the CTMS design process included the definition of the system architecture and data interchange mechanisms, selection of storage media, database modeling and standardization, user interface (UI) and user experience (UX) design, and development of security and privacy policies based on analyzing and categorizing the requirements collected in the first phase, referring to good clinical practice (GCP) and the guidance documents of the National Medical Products Administration (NMPA). The functions and data required by each participant in the clinical trial are determined. Figure 1 shows the system architecture of the CTMS, which is divided into seven subsystems to meet the corresponding requirements of the first stage. Seven dimensions were put forward, including the clinical trial project management system (CTPMS), clinical trial ethical management system (CTEMS), clinical trial subject management system (CTSMS), clinical trial investigational product management system (CTIPMS), clinical trial financial management system (CTFMS), clinical trial quality management system (CTQMS), and permission management and maintenance system (PMMS). These systems include the functions of the whole-process management of clinical trials. The unified control of permissions is realized through single sign-on (SSO) between the systems. To ensure a more efficient operation of the CTMS, it is necessary to focus on the data integration mode with the hospital clinical and business systems (i.e., HIS, LIS, EMR, CDR, etc.).
The unified management of interface services is defined, as shown in Fig. 1, as a process of complete data integration, which primarily involves three types of data service functions: (1) basic data synchronization, which serves as the basis for system operation; (2) real-time data query to meet the needs of subject information retrieval and clinical trial data monitoring; and (3) diagnosis and treatment data generated by the CTMS, which are transmitted to the clinical business system of the hospital to meet the continuity requirement of diagnosis and treatment of the subjects. Database modeling and standardization are performed with reference to the Clinical Data Interchange Standards Consortium (CDISC) to obtain a standard vocabulary to ensure the integrity of the data model; in terms of system security and subject privacy protection, security protection strategies and development specifications were formulated with reference to the Health Insurance Portability and Accountability Act (HIPAA) and China’s privacy protection laws. In addition, the CTMS implements fine-grained isolation and verification of permissions, and it records all events in a log to ensure the traceability of the data.
|
Third stage
The third stage focused on the development and testing of the CTMS. This was divided into two steps according to the definition of the design stage. The first step realizes clinical trial approval and review management, and the developed subsystems include CTPMS, CTEMS, and PMMS, which focus on the process management of the study project application and review stage, and do not involve data integration with external systems. The second step realizes clinical trial operational management. The developed subsystems include CTSMS, CTIPMS, CTFMS, and CTQMS, which complete internal function development and data integration with external systems, and realize the entire process of data management. The CTMS was developed based on the distributed architecture of the Java programming language, and its database is Oracle Database 11 g Release 2 on a server running the Linux CentOS 7.6 operating system; data integration with external systems is managed through an interface services unified management platform. The system adopts browser/server architecture and supports current mainstream browsers, such as Google Chrome, Internet Explorer, and Firefox Browser for encrypted access via hypertext transfer protocol secure (HTTPS). System testing mainly includes verification of functional effectiveness, data security, system reliability, and the efficiency of data integration with external systems.
Fourth stage
This stage involved CTMS deployment and system operation training. In November 2014, CTPMS, CTEMS, and PMMS were officially launched, realizing clinical trial approval and review management in FAHZU. In March 2015, the development of CTSMS, CTMMS, CTFMS, and CTQMS was completed. Considering that the clinical trial operational management involves data integrity verification and data integration with external systems, a new pilot run phase was needed. By selecting a certain number of clinical trials in Phases I–IV, the operating results were used to verify the compatibility of the system with different types of clinical trials. The CTMS pilot run system first selects Phase I clinical trials and runs a total of 20 projects. Through continuous updating of functions during operation, it can fully meet the needs of Phase I clinical trial operational management. Subsequently, it selects clinical trials in Phases II–IV. A total of 10 projects have been run, and the functions have been stable and meet the requirements of various types of clinical trials. However, training hospital staff in the use of CTMS is critical. Through a variety of methods including face-to-face training and offline data learning, all staff related to clinical trials have been trained. The first version of CTMS was fully launched in FAHZU in September 2015.
Results
FAHZU is the first hospital in China to independently develop an integrated CTMS; the system has been successfully implemented in the hospital since September 2015. As of March 2021, the hospital now runs an updated version of CTMS known as V4.2.5. The data management of the entire process of clinical trials from project approval and review management to operational management has been fully realized. The FAHZU CTMS operates independently by design as a fully functional system at the application level (not as a component of the HIS), establishes a dedicated clinical trial database at the data level, and completes data integration with external systems through a unified interface system, to achieve process continuity and data integrity. Through the construction of an integrated CTMS, the flow of multi-party collaboration tasks is optimized, and non-research matters such as finance and data processing are simplified, to effectively improve the efficiency and quality of clinical trials. The results shown below are based on the latest stable version of FAHZU CTMS, V4.2.5.
System categories and features for clinical trial
The first level involves clinical trial project approval and review management, and its functions cover the rationality review of clinical trial study projects and related affairs management by CTI and EC. Table 2 lists the categories and features of clinical trial approval and review management, and the services provided to users through CTPMS and CTEMS. Clinical trial project approval and review management are based on multi-role collaboration, focusing on improving efficiency as the core and supporting remote project application, full electronic project approval, review of process approval documents online, aggregation of reviews into review comments, and timely generation of tasks and notification. The main features of CTPMS include project approval management, to-do task list, project list, document management, contract management, initial meeting management, investigator management, and CRC management. Through the organic combination of these features, the CTI realizes the project approval review and daily management of study projects. The main features of CTEMS include EC management, ethics review management, and ethics conference management, which manages the continuous ethics review of study projects by the EC, including initial protocol review, protocol amendment review, SAE report and review, and violation/deviation protocol review.
| ||||||||||||||||||||||||||||||
The second level comprises clinical trial operational management, which is a complex and continuous management process. Table 3 lists the categories and features of clinical trial operational management; the system provides services to users through the CTSMS, CTIPMS, CTFMS, and CTQMS. CTSMS is mainly composed of three stages: operational management pre-configuration, subject recruitment, and subject visit management. The features included in the operational management pre-configuration stage include study participant assignments, protocol configuration, electronic case report form (e-CRF) design, rule configuration, and global control over the access rights and visitation rules of subjects under the study project. The main features of the subject recruitment stage of the system include subject recruitment, subject violation verification (such as determining whether the subject is participating in another clinical trial and inputting incorrect information of subject), subject lists, and subject global labeling, to realize the registration of subjects under the corresponding study project, status labeling, and visualization.
There are three main events in the subject visit management stage, which are described as follows:
- Obtain the visit content information in the corresponding study cycle according to the study plan and turn it into a to-do list. The visit content includes subject screening, inspection/examination issuance, prescription issuance, treatment, randomization of subjects, e-CRF filling, and AE/SAE reporting.
- Enter operational status changes of subjects, including switching protocols, admission, or discharge, entering the next visit stage, and status of subject updates (dropping out, withdrawal, failure to follow up, etc.).
- Complete data queries of subjects, including outpatient/inpatient medical records, inspection/examination results queries, subject fee queries, and e-CRF data filling.
CTIPMS adopts the central management model, which is managed by qualified personnel designated by the CTI. Through the organic combination of stock management, prescription management, label management, and intelligent detection, closed-loop management of the entire process of trial investigational products is realized. CTFMS primarily combines the financial characteristics of clinical trials and the relevant requirements of the hospital’s financial management, including payment and allocation, budget management, expenditure management, workload statistics, and project funding amounts to achieve orderly financial management based on greatly reducing researchers’ time consumption. Similarly, CTQMS mainly includes regular quality control data report generation and reporting, as well as dynamic quality control based on collected data.
| ||||||||||||||||||||||||||||||||||||||||||||||||||
The third level is backstage management, which is indispensable for the stable operation of the CTMS. Table 4 shows the categories and features for backstage management of clinical trials. In this level, the proposed system provides services to users through the PMMS. Unified management of all user information and data access permissions of the system through user and permission management provides a basis for the realization of the collaboration of users with different roles. Log management identifies the problems in the system operation and records all the data changes for verification. These two points are also an indispensable part of achieving secure data access. Data statistics are recorded according to the requirements of CTI to provide managers with statistical charts of study projects and operational data to provide decision support capability.
| |||||||||||||||||||||||||||
Enterprise process management for clinical trials
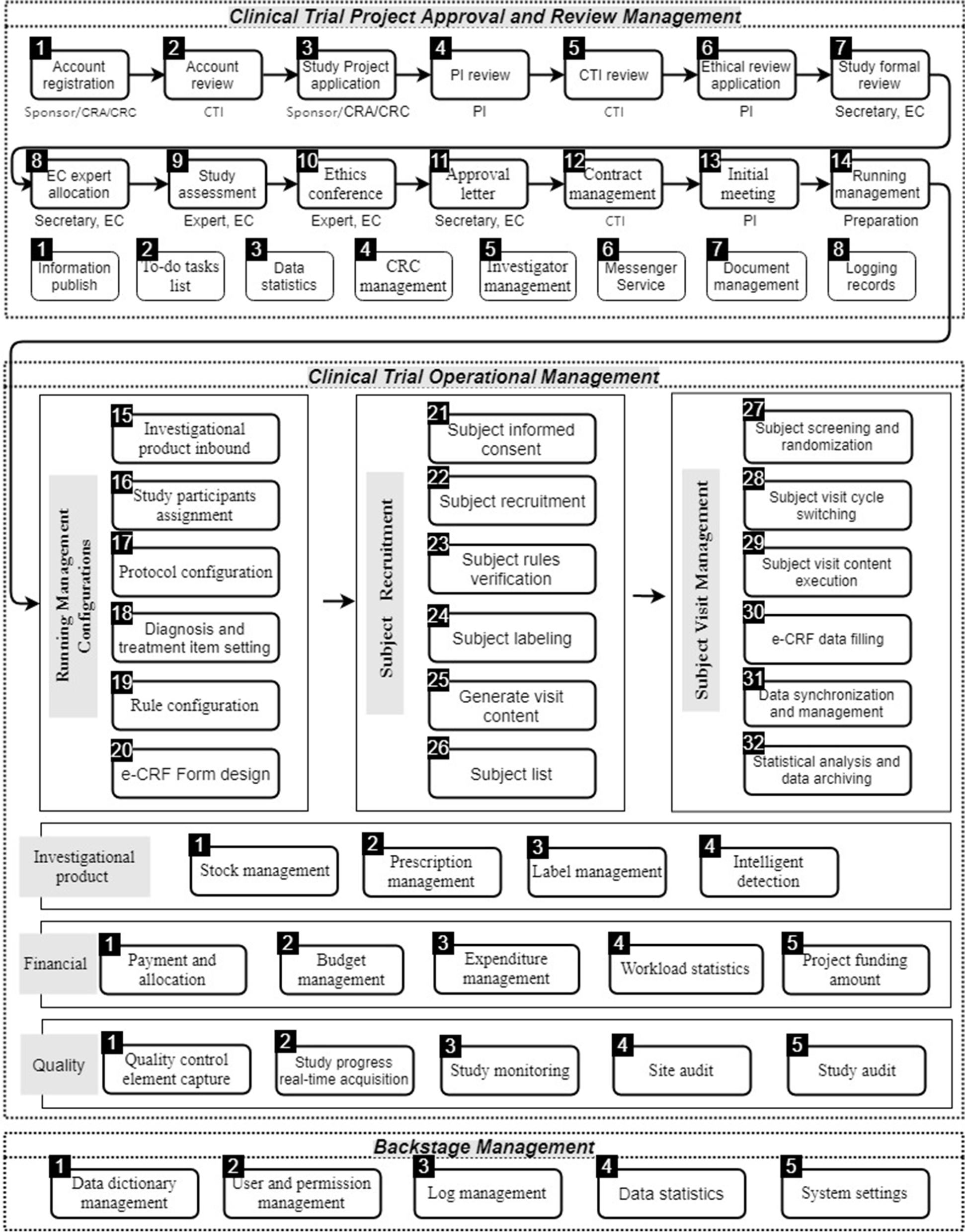
FAHZU CTMS realizes the entire process management of clinical trials through the organic combination of project approval and reviews management, clinical trial operational management, and backstage entire process management, which is further categorized into 59 internal processes by combining user and authority management (Fig. 2). Clinical trial project approval and review management consists of 14 main processes and eight auxiliary processes, which can efficiently complete the review and contract signature phases of the study project; a kick-off meeting is held to allow entry into clinical trial operational management. Clinical trial operational management is a complex and long-term process involving multiple factors such as subjects, diagnosis and treatment, medicine, and finance. We divide it into six stages: operational management pre-configuration, subject recruitment, subject visit management, investigational product management, financial management, and quality management, and then further subdivide these stages into 32 internal processes. Finally, backstage management, as the basic component, supports the stable operation of the entire clinical trial process.
|
Data integration with external systems
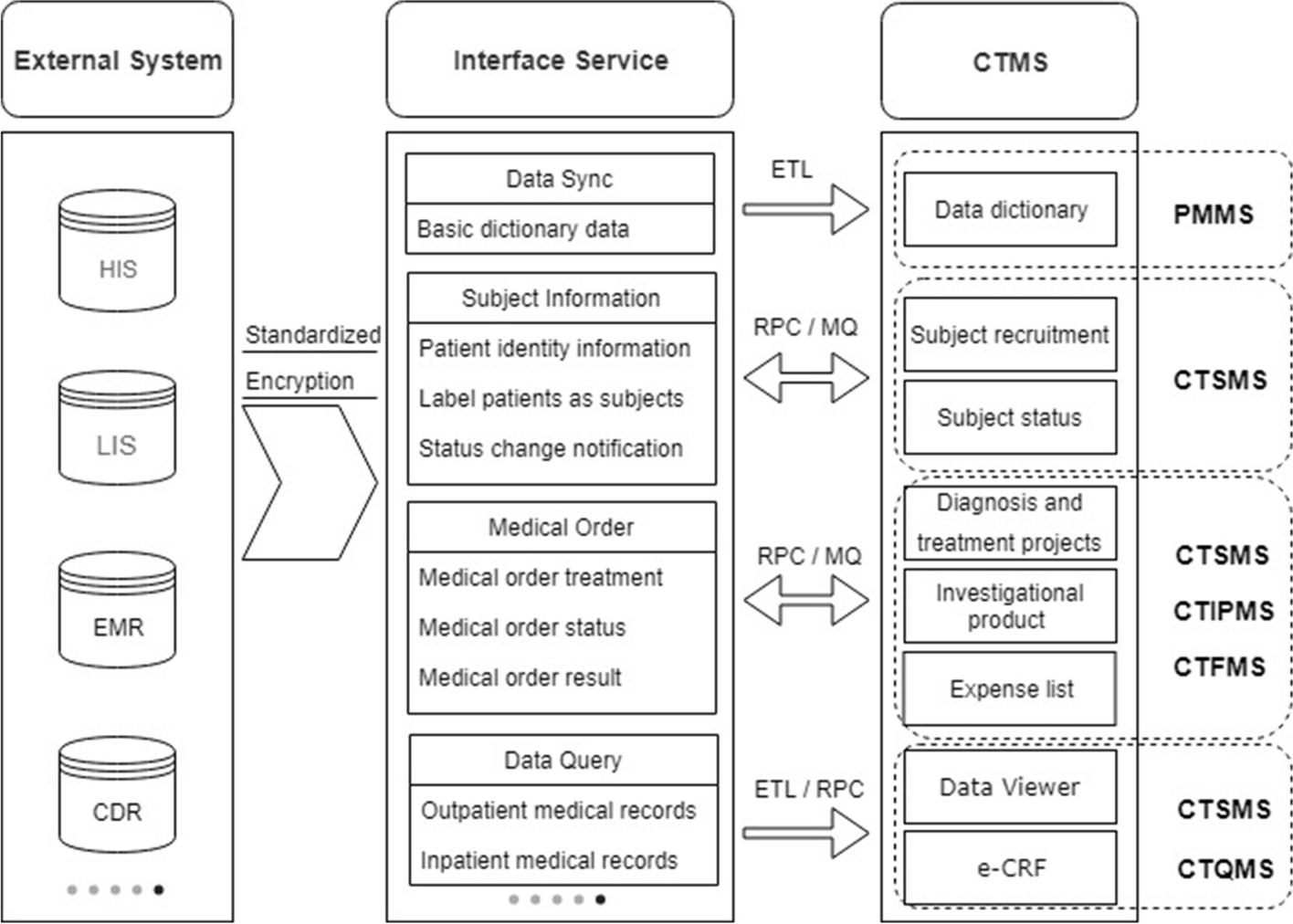
To use the CTMS as a dedicated database for clinical trials, data integration with external systems is indispensable. Figure 3 shows some of the critical data integration services between the CTMS and external systems. CTMS subsystems integrate with external systems such as HIS, LIS, EMR, and CDR through the interface service platform to achieve data service standardization and transmission process encryption. The data integration method supports extract-transform-load (ETL), message queue (MQ), and remote procedure call (RPC) services and is flexibly selected based on the data volume and business model. Data synchronization and data queries involve a large amount of data. The operations use ETL batch extraction and RPC service real-time queries, which is used for data dictionary maintenance, as well as queries of subjects’ full diagnosis and treatment data, and e-CRF structured data filling. Subject information data and medical order data use the synchronous call of RPC service and asynchronous notification of MQ. Synchronous calling is used to obtain the patient information of the subject recruitment, the subject’s diagnosis and treatment items, and prescription issuance, billing, etc. Asynchronous calling is used for notification of changes in the subject’s status, obtaining medical order status, obtaining results, etc.
|
Data security and privacy protection
Data security and privacy protection ensure that a mature process is developed and maintained throughout the design life cycle. Data security primarily involves data storage and data access security. Privacy protection focuses on ensuring data security while complying with laws and regulations to de-identify sensitive patient information.
Data security mainly includes the two aspects of data storage security and data access security. In terms of data storage security, FAHZU CTMS adopts two strategies: (1) multi-node storage of data to achieve high availability of data services and a data backup strategy to ensure that data will not be lost in case of failure, and (2) encrypted storage of sensitive data (e.g., passwords, ID numbers, and bank card numbers). Policies for data access security are:
- Security at the network level, through safety equipment (such as a firewall, host security protection, bastion host, and database/log audit tools), to implement access control and prevent illegal access;
- Security at the application level through multiple roles of authority management system access controls on data content, especially including rules to ensure no open access to prescriptions (e.g., CRC non-prescription issuance authority), as well as finding and repairing the vulnerabilities of the application through penetration testing and completing the information security technology-evaluation requirement for classified protection of cybersecurity—Level 3; and
- Maintaining a detailed data change log can be useful in tracing the record of the process of data recovery.
Subject privacy protection aims to protect sensitive patient information through a series of de-identification processes, which are explicitly required in the GCP. The FAHZU CTMS strictly implements the requirements of subject privacy protection in the GCP guidelines and refers to the Information Security Technology Personal Information Security Specification issued by the Standardization Administration of China and the HIPAA Regulations issued by the U.S. Department of Health. Final compliance with HIPAA’s implementation specifications requiring de-identification of protected health information excludes 18 personal health identifiers (PHIs) from the CTMS (e.g., name, address, cell phone number, and social security number) to comply with international and national laws. For CTMS users to identify subjects, we used the clinical trial protocol number, subject screening number, and subject name initials to uniquely identify them. At the level of data interaction, the patients’ medical record numbers in the hospital are systematically encrypted and stored in the CTMS database, which is linked to the data of the external clinical and business systems (i.e., HIS, LIS, EMR, CDR, etc.) for automatic data transmission.
Evaluation
The evaluation of the CTMS is ongoing and mainly conducted at two levels: clinical trial project approval and review management, and clinical trial operational management. The former focuses on the convenience of multi-party collaboration and the efficiency of project approval, whereas the latter focuses on the evaluation of subject-centered data management throughout the process and the improvement of the quality of clinical trials.
The improved clinical trial project approval and review management after applying the FAHZU CTMS, compared with that before the system was implemented, mainly embodies two distinct advantages. First, it realizes process automation management based on task and, combined with workflow technology, cooperates with user authority management systems, realizes automatic triggering of node events, and performs automatic assignment of processing personnel and message notifications such that personnel only need to process their personal to-do lists according to the message reminder, reducing the complexity of system use under multi-party collaboration. Second, clinical trial project approval supports remote application, and electronic document management enables online submission of project approval materials, as well as online review and summary of revision opinions in the review process, which reduces the workload of project approval materials review and improves the efficiency of project review. Since CTMS began to operate in the hospital in December 2014, 1,388 study projects have been accepted and 43,051 documents have been submitted through CTMS as of March 31, 2021.
The focus at the clinical trial operational management level is centered on patient outcomes to achieve full-cycle data management. Here, the advantages are more significant. The four core advantages are summarized as follows:
- The CTMS requires that the research plan must be entered before the recruitment of subjects, and the visit content of the current visit cycle can be automatically correlated during the subject visit. It is transformed into to-do tasks in the current research stage, with timely reminders, reducing deviations from the protocol.
- An independent billing model is adopted for clinical trial-related inspection and treatment expenses so that subjects can be exempted from expense reimbursement and be marked in the hospital business system to meet non-clinical trial diagnosis and treatment reminders and clinical trial inspection green special requirements such as channels.
- The CTMS contains the data on each subject, including newly generated data during operation and data collected in the hospital business system. Through strict authority classification, the scope of the data queries, such as those issued by the CRC and other data query authorities, are limited to subjects who are responsible for the project.
- Clinical trial drugs are label-based full-cycle closed-loop managed, and transfers are completed through label scanning, and support some special properties of clinical trial drugs, such as open, single-blind, and double-blind studies, and situations with other prescriptions, where drugs need to be random, and when the serial numbers and medicine need to be recalled.
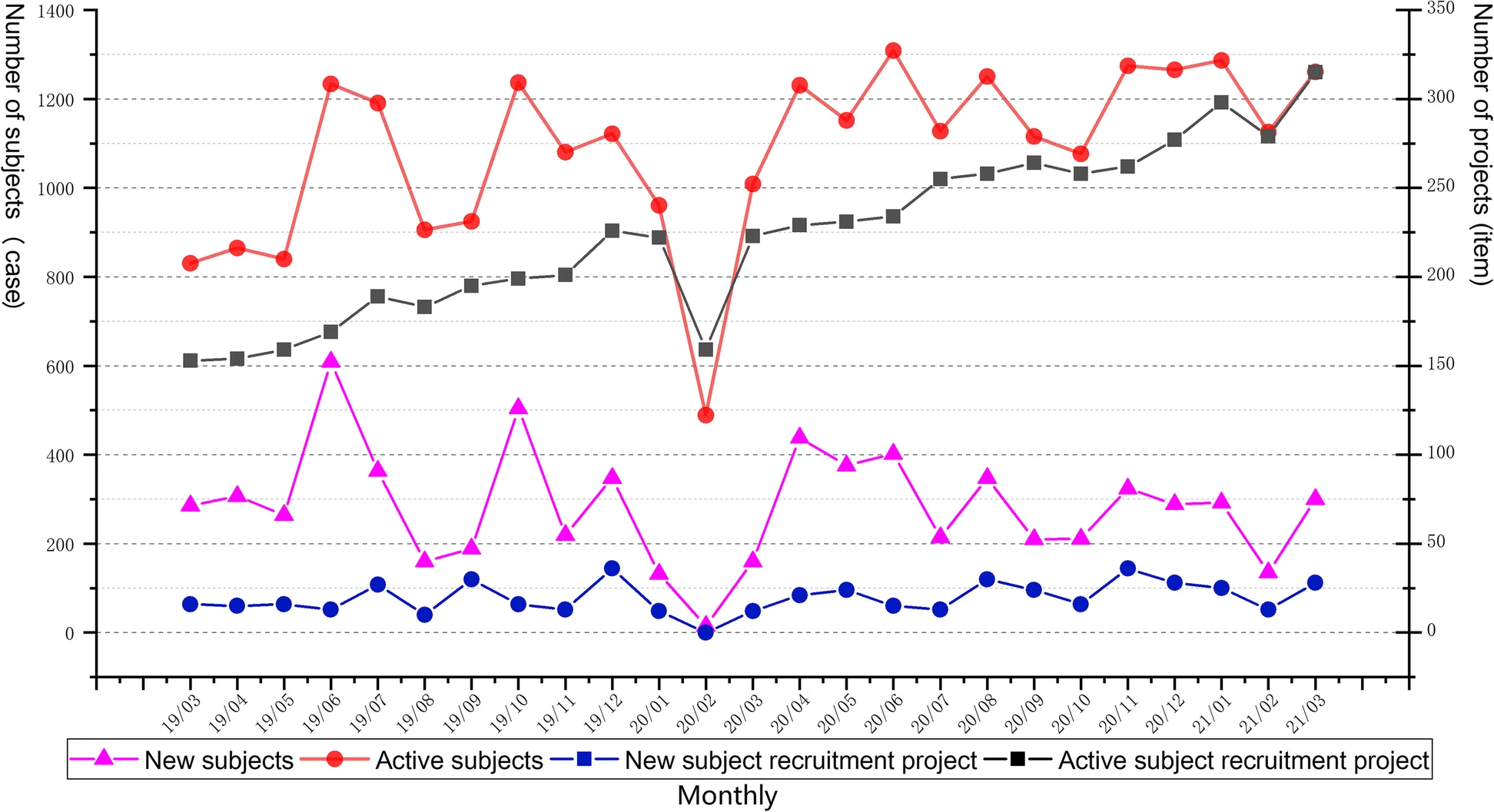
The clinical trial operational management of CTMS began a pilot run at FAHZU in March 2015. Pilot runs are conducted to validate and improve the effectiveness of system functions and compatibility with different types of clinical trials. Since September 2015, all newly initiated clinical trials on the site have been managed through CTMS. According to statistics, as of March 31, 2021, a total of 12,144 subjects have been included in the management of CTMS, across 472 study projects. Figure 4 shows the change in the number of subjects and corresponding study projects enrolled in CTMS in the last two years. We observed that the monthly number of new subjects and the corresponding study projects remained stable, and the number of active subjects also remained stable, while the corresponding monthly number of active projects increased. Since the outbreak of COVID-19 in China in December 2019, with the support of the integrated CTMS and necessary measures (e.g., remote follow-up, express delivery, and remote inspection), it may be observed from Fig. 4 that the clinical trials have continued at a steady rate during the COVID-19 outbreak.
|
Discussion
Digitalization is an inevitable trend in clinical trial management.[9] At present, there are many electronic clinical trial management systems in China; however, there are few systems that can connect with the information system of the clinical research center and unify the sponsor, data management department, and other parties into a platform for cooperation. The FAHZU CTMS has been researched and developed fully independently based on a distributed service architecture. It takes process management and trial data as the core; highly integrates, interconnects, and interfuses with hospital clinical business systems; and combines key contents such as data security and privacy protection to achieve independent application layers and interconnected data layers. Thus, comprehensive process management and dynamic real-time monitoring of clinical trials can be realized. Compared with commercial products, our self-developed CTMS is more cost-effective and highly customizable, fully fits the management needs of the hospital as a clinical trial administrative institution, and allows more timely system version updates, system operation, and maintenance response. Based on the deep understanding of hospital clinical business systems such as HIS, LIS, CDR, PACS, and EMR by information technology experts, a more comprehensive scheme was designed to achieve a high degree of integration between the CTMS and clinical business systems. The large amounts of medical data required for clinical trials are docked into the developed system and used as a CTMS-independent data collection and storage subsystem. In addition, based on the full understanding of data security and privacy protection, the security of the system was assigned great importance from the beginning of system development, and the privacy protection function of subjects was improved to better serve the entire process management of clinical trials in our institution.
From an operational point of view, the use of the system has significantly improved the efficiency of researchers and institutional managers. For the institutional management, the system realizes real-time and efficient management of the entire process of clinical trials and ensures the reliability, authenticity, and integrity of clinical trial results. To ensure the safety of subjects, it realizes limited sharing of information through the data query, statistics, and tracking module. Institutional managers can view the project schedule and browse project-related node information, to accurately grasp the implementation of the project status, improve the quality of clinical trial data, reduce time consumption, and promote the standardized implementation of clinical trials according to the GCP guidelines and SOP of our hospital. On this basis, a full-cycle data quality monitoring and early warning platform for clinical trials can be constructed. Through artificial intelligence (AI) technologies such as text mining, natural language processing, machine learning, and knowledge mapping, intelligent full-cycle data analysis and early warning in clinical trials can be realized. These include the matching of inclusion and exclusion criteria, warning of inspection, warning of combined drug prohibition, warning of absence of visit activity or out of window, and intelligent warning of underreporting of AE or SAE.
The protection of private information is a more important consideration than the system construction.[10][11][12] The biggest problem with the establishment of the system is that the subject information may be exposed, which is a serious ethical problem. To solve potential risks from the level of laws and regulations, it is necessary to think deeply. Therefore, at the beginning of the design, we paid special attention to the concept of network data security and privacy protection, carried out the privacy impact assessment, and integrated the measures of privacy protection into the entire process of information system development. Institutional managers, researchers, quality controllers, CRCs, drug administrators, and inspectors are divided into different users. There is a strict permission management system for user accounts, to avoid the problem of account borrowing, certificate authority authentication or face recognition systems should be added to adopt fine-grained permission management. In addition, the SOP for risk assessment is particularly important, including the definition, classification, and rating of risks.[13] Only information management systems that meet the requirements of risk assessment can be operated. In the information age, everything is connected, and information is a trend.[14][15] In today's highly developed internet technology, we need to think about and solve the problems of data security and personal privacy, as well as the specific procedures to improve the efficiency of clinical research. At the same time, compliance with the Chinese GCP and International Conference on Harmonisation (ICH) requirements for GCP ensure compliance with ethical and legal provisions.
Compared with the traditional paper CRF, e-CRF allows researchers to enter the CRF electronically based on the source data rather than fill it in manually.[12] However, source data verification (SDV) is still required to compare the CRF with original medical records and inspection lists to ensure the quality of data input. Our CTMS is now able to automate the collection of structured data, eliminate the SDV process, and further simplify the process. Thus, any modifications to the electronic source data can be recorded through an audit trail. In the future, our system will also study the complex natural language analysis involved in unstructured data. Simultaneously, we will build a way to connect electronic data acquisition (EDC) and hospital e-CRF to directly collect and transfer clinical data electronically, ensuring the quality and integrity of the data.
In the future, we also want to utilize the data in the clinical trial management platform to realize intelligent subject recruitment and improve the efficiency of subject recruitment. Recruiting subjects for some clinical trials is difficult, especially those involving rare diseases, stringent admission criteria, and special subgroups. Owing to information asymmetry, researchers only know the condition of the patients they are treating, and patients do not know that their disease may be under research at the hospital, which can seriously affect the progress of clinical trials.[16][17][18][19] To fully utilize the hospital CTMS system of clinical data, the central hospital and administering medical hospital can simultaneously be on the same clinical trial management platform, which will further enrich the patient resources. Additionally, the system can provide search functions, select exclusion criteria, fast-matching potential subjects, and realize intelligent recruitment of subjects, which can effectively help the sponsor accelerate the clinical trial process, reduce R&D costs, and successfully seize market opportunities. However, in the era of big data, these data have important scientific value in the field of clinical research. Applications such as large data analysis found that local residents’ disease condition, and its influencing factors—specific studies on key diseases—have been successfully applied to determine the time of disease distribution, location distribution, population distribution, and analysis of risk factors of disease; furthermore, it has been used for the evaluation of the effectiveness of clinical screening and diagnosis methods, inspection treatment or drug treatment effect, optimization of individual diagnosis and treatment of disease, and research on the influencing factors of diseases after intervention. This is to provide the basis for the local health administration departments to make health management decisions.
Since the outbreak of COVID-19, many jobs around the world have been stalled to a certain extent. In the event of a major public health emergency, carrying out clinical trials and ensuring the smooth implementation of monitoring work has become a problem for clinical trial practitioners, and remote monitoring has therefore been put on the agenda. The U.S. Food and Drug Administration (FDA) has long encouraged more centralized monitoring, where inspectors perform inspections in the office using relevant information tools rather than at research institutions (hospitals). The development trend of clinical trial monitoring is to replace on-site monitoring with centralized monitoring.[20] Remote monitoring can improve the quality and efficiency of clinical research and reduce its cost, and it is possible only when a series of electronic clinical trial products such as CTMS, electronic data acquisition, EMR, and clinical data management systems are widely used. The clinical trial information management system of our hospital is a CTMS that unites multiple teams on one platform and is highly integrated with all clinical systems of the hospital. As a web-based platform, remote data monitoring and cloud auditing can be included on the CTMS as a mature operation.
Compared with other information systems, CTMSs are more professional and personalized. The realization of the effectiveness of a CTMS requires a significant amount of time, and the more clinical trials undertaken, the more significant the effect. Compared with the system design, the comprehensive and efficient application of the system takes longer to achieve. With the digitization of clinical research, the sharing and integration of research data will bring many management advantages, such as an increase in available management resources, scientific and data support for major decisions, the convenience of remote management, pertinacity, and pre-operation. The CTMS developed by our hospital will be constantly updated and upgraded, and its functions will constantly improve. The system update will keep pace with developments in international drug clinical trial management, promote the development of clinical trials in a more standardized direction, and promote the disciplinary status of the hospital regarding high-level clinical trials.
Conclusion
The FAHZU CTMS, as the first integrated CTMS independently developed by a hospital in China, can better adapt to the institutional needs for individualized, whole-process, and dynamically comprehensive evaluation and supervision of clinical trials. The integrated CTMS contains three levels and seven subsystems, which fully realizes the whole-process data management of clinical trials from project approval and review management to operational management. Through the unified interface system, the developed CTMS provides a variety of access methods to complete efficient data integration with the clinical business systems, and applies multiple security policies combined with privacy protection methods to effectively ensure the security of data and the privacy of subjects during clinical trial operation. The operation results based on the integrated CTMS show that it can effectively control the risks in the clinical trial process, so as to improve the science, safety, and timeliness of the new drug development process.
Abbreviations, acronyms, and initialisms
- AE: adverse event
- AI: artificial intelligence
- CDISC: Clinical Data Interchange Standards Consortium
- CDR: clinical data repository
- CRA: clinical research associate
- CRC: clinical research coordinator
- CTEMS: clinical trial ethical management system
- CTFMS: clinical trial financial management system
- CTI: clinical trial institution
- CTIPMS: clinical trial investigational product management system
- CTMS: clinical trial management system
- CTPMS: clinical trial project management system
- CTQMS: clinical trial quality management system
- CTSMS: clinical trial subject management system
- e-CRF: electronic case report form
- EC: ethics committee
- EMR: electronic medical record
- ETL: extract-transform-load
- FAHZU: First Affiliated Hospital, Zhejiang University School of Medicine
- CP: good clinical practice
- HIPAA: Health Insurance Portability and Accountability Act
- HIS: hospital information system
- HTTPS: hypertext transfer protocol secure
- ICH: International Conference on Harmonisation
- LAN: local area network
- LIS: laboratory information system
- PACS: picture archiving and communication systems
- PHI: personal health identifier
- PI: principal investigator
- PMMS: permission management and maintenance system
- R&D: research and development
- RPC: remote procedure call
- SAE: serious adverse events
- SDV: source data verification
- SSO: single sign-on
- UI: user interface
- UX: user experience
Acknowledgements
Author contributions
LS conceived the concept of the project, was responsible for the architecture and integration concept, designed and developed the system, and wrote the manuscript. YZ collected and sorted out the requirements for the construction of the clinical trial management system and collected references. AXP and QWZ designed and organized the tables and figures in the article. MZ and JL were responsible for conceptualization and formal analysis, reviewed the manuscript, and supplemented the discussion section. All authors read and approved the final manuscript.
Ethics approval and consent to participate
There were no individual-level data for the study, so ethics committee approval was not required. All executive trials included in this study system were the Clinical Trial Ethics Committee (EC) of The First Affiliated Hospital, Zhejiang University School of Medicine (FAHZU) reviewed and approved. All subjects in these trials signed informed consents prior to enrollment.
Funding
This study was funded by the New Drug Creation Project of The 13th Five-Year National Science and Technology Major Special Project (2020ZX09201-003).
Availability of data and materials
FAHZU CTMS consists of several subsystems. Considering data security and privacy protection, subsystems associated with subject data are deployed based on the hospital’s internal network, and domain names are resolved by a self-built domain name systemserver. The following four links show part of the web pages of the core subsystem, which can show the function design and data volume of the system. For additional information please contact the corresponding author.
1. https://doi.org/10.5281/zenodo.5880663 2. https://doi.org/10.5281/zenodo.5880783 3. https://doi.org/10.5281/zenodo.5880799 4. https://doi.org/10.5281/zenodo.5880826
Software availability and requirements
Project name: FAHZU CTMS Project home page: https://ctms.zy91.com Operating system(s): Platform-independent Programming language: Java Other requirements: Java 1.7.1 or higher, Tomcat 7.0 or higher, Nginx 1.14.2 or higher, Oracle Database 11 g Release 2, Redis 4.0 License: Free for academics Any restrictions to use by non-academics: Contact authors
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- ↑ Kruizinga, M. D.; Stuurman, F. E.; Exadaktylos, V.; Doll, R. J.; Stephenson, D. T.; Groeneveld, G. J.; Driessen, G. J. A.; Cohen, A. F. (1 October 2020). Michel, Martin. ed. "Development of Novel, Value-Based, Digital Endpoints for Clinical Trials: A Structured Approach Toward Fit-for-Purpose Validation" (in en). Pharmacological Reviews 72 (4): 899–909. doi:10.1124/pr.120.000028. ISSN 0031-6997. http://pharmrev.aspetjournals.org/lookup/doi/10.1124/pr.120.000028.
- ↑ Davi, Ruthie; Mahendraratnam, Nirosha; Chatterjee, Arnaub; Dawson, C. Jill; Sherman, Rachel (1 December 2020). "Informing single-arm clinical trials with external controls" (in en). Nature Reviews Drug Discovery 19 (12): 821–822. doi:10.1038/d41573-020-00146-5. ISSN 1474-1776. https://www.nature.com/articles/d41573-020-00146-5.
- ↑ the COVID19 and Cancer Clinical Trials Working Group; Desai, Aakash; Gainor, Justin F.; Hegde, Aparna; Schram, Alison M.; Curigliano, Giuseppe; Pal, Sumanta; Liu, Stephen V. et al. (1 May 2021). "Author Correction: COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials" (in en). Nature Reviews Clinical Oncology 18 (5): 320–320. doi:10.1038/s41571-021-00503-2. ISSN 1759-4774. PMC PMC7985918. PMID 33758378. https://www.nature.com/articles/s41571-021-00503-2.
- ↑ Manem, Venkata S.K.; Salgado, Roberto; Aftimos, Philippe; Sotiriou, Christos; Haibe-Kains, Benjamin (1 October 2018). "Network science in clinical trials: A patient-centered approach" (in en). Seminars in Cancer Biology 52: 135–150. doi:10.1016/j.semcancer.2017.12.006. https://linkinghub.elsevier.com/retrieve/pii/S1044579X17302365.
- ↑ Tan, Eng-King (1 January 2021). "Movement disorders in 2020: clinical trials, genetic discoveries, and COVID-19" (in en). The Lancet Neurology 20 (1): 10–12. doi:10.1016/S1474-4422(20)30448-8. PMC PMC7833604. PMID 33340472. https://linkinghub.elsevier.com/retrieve/pii/S1474442220304488.
- ↑ Bhagat, Seema; Kapatkar, Vaibhavi K.; Mane, Ashish; Pinto, Colette; Parikh, Devang; Mittal, Gaurav; Jain, Rishi (14 February 2020). "An Industry Perspective on Risks and Mitigation Strategies Associated with Post Conduct Phase of Clinical Trial" (in en). Reviews on Recent Clinical Trials 15 (1): 28–33. doi:10.2174/1574887114666191016103332. http://www.eurekaselect.com/175736/article.
- ↑ Nourani, Aynaz; Ayatollahi, Haleh; Dodaran, Masoud Solaymani (30 January 2019). "A Review of Clinical Data Management Systems Used in Clinical Trials" (in en). Reviews on Recent Clinical Trials 14 (1): 10–23. doi:10.2174/1574887113666180924165230. http://www.eurekaselect.com/165619/article.
- ↑ Park, Yu Rang; Yoon, Young Jo; Koo, HaYeong; Yoo, Soyoung; Choi, Chang-Min; Beck, Sung-Ho; Kim, Tae Won (24 April 2018). "Utilization of a Clinical Trial Management System for the Whole Clinical Trial Process as an Integrated Database: System Development" (in en). Journal of Medical Internet Research 20 (4): e103. doi:10.2196/jmir.9312. ISSN 1438-8871. PMC PMC5941091. PMID 29691212. http://www.jmir.org/2018/4/e103/.
- ↑ Nourani, Aynaz; Ayatollahi, Haleh; Dodaran, Masoud Solaymani (21 August 2019). "Clinical Trial Data Management Software: A Review of the Technical Features" (in en). Reviews on Recent Clinical Trials 14 (3): 160–172. doi:10.2174/1574887114666190207151500. http://www.eurekaselect.com/169754/article.
- ↑ Barlow, Candida (1 April 2020). "Human Subjects Protection and Federal Regulations of Clinical Trials" (in en). Seminars in Oncology Nursing 36 (2): 151001. doi:10.1016/j.soncn.2020.151001. https://linkinghub.elsevier.com/retrieve/pii/S0749208120300164.
- ↑ Barlow, Candida (1 April 2020). "Oncology Research: Clinical Trial Management Systems, Electronic Medical Record, and Artificial Intelligence" (in en). Seminars in Oncology Nursing 36 (2): 151005. doi:10.1016/j.soncn.2020.151005. https://linkinghub.elsevier.com/retrieve/pii/S0749208120300206.
- ↑ 12.0 12.1 Finniss, Damien G; Kaptchuk, Ted J; Miller, Franklin; Benedetti, Fabrizio (1 February 2010). "Biological, clinical, and ethical advances of placebo effects" (in en). The Lancet 375 (9715): 686–695. doi:10.1016/S0140-6736(09)61706-2. PMC PMC2832199. PMID 20171404. https://linkinghub.elsevier.com/retrieve/pii/S0140673609617062.
- ↑ Jørgensen, Lars; Paludan-Müller, Asger S.; Laursen, David R. T.; Savović, Jelena; Boutron, Isabelle; Sterne, Jonathan A. C.; Higgins, Julian P. T.; Hróbjartsson, Asbjørn (1 December 2016). "Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews" (in en). Systematic Reviews 5 (1): 80. doi:10.1186/s13643-016-0259-8. ISSN 2046-4053. PMC PMC4862216. PMID 27160280. http://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-016-0259-8.
- ↑ Cowie, Martin R.; Blomster, Juuso I.; Curtis, Lesley H.; Duclaux, Sylvie; Ford, Ian; Fritz, Fleur; Goldman, Samantha; Janmohamed, Salim et al. (1 January 2017). "Electronic health records to facilitate clinical research" (in en). Clinical Research in Cardiology 106 (1): 1–9. doi:10.1007/s00392-016-1025-6. ISSN 1861-0684. PMC PMC5226988. PMID 27557678. http://link.springer.com/10.1007/s00392-016-1025-6.
- ↑ Sharma, Abhinav; Harrington, Robert A.; McClellan, Mark B.; Turakhia, Mintu P.; Eapen, Zubin J.; Steinhubl, Steven; Mault, James R.; Majmudar, Maulik D. et al. (1 June 2018). "Using Digital Health Technology to Better Generate Evidence and Deliver Evidence-Based Care" (in en). Journal of the American College of Cardiology 71 (23): 2680–2690. doi:10.1016/j.jacc.2018.03.523. https://linkinghub.elsevier.com/retrieve/pii/S0735109718344139.
- ↑ Kempf, Lucas; Goldsmith, Jonathan C.; Temple, Robert (1 April 2018). "Challenges of developing and conducting clinical trials in rare disorders" (in en). American Journal of Medical Genetics Part A 176 (4): 773–783. doi:10.1002/ajmg.a.38413. ISSN 1552-4825. https://onlinelibrary.wiley.com/doi/10.1002/ajmg.a.38413.
- ↑ Brasil, Sandra; Pascoal, Carlota; Francisco, Rita; dos Reis Ferreira, Vanessa; A. Videira, Paula; Valadão, Gonçalo (27 November 2019). "Artificial Intelligence (AI) in Rare Diseases: Is the Future Brighter?" (in en). Genes 10 (12): 978. doi:10.3390/genes10120978. ISSN 2073-4425. PMC PMC6947640. PMID 31783696. https://www.mdpi.com/2073-4425/10/12/978.
- ↑ Wu, Jasmanda; Wang, Cunlin; Toh, Sengwee; Pisa, Federica Edith; Bauer, Larry (1 October 2020). "Use of real‐world evidence in regulatory decisions for rare diseases in the United States—Current status and future directions" (in en). Pharmacoepidemiology and Drug Safety 29 (10): 1213–1218. doi:10.1002/pds.4962. ISSN 1053-8569. https://onlinelibrary.wiley.com/doi/10.1002/pds.4962.
- ↑ Groft, Stephen C.; Posada de la Paz, Manuel (2017), Posada de la Paz, Manuel; Taruscio, Domenica; Groft, Stephen C., eds., "Preparing for the Future of Rare Diseases", Rare Diseases Epidemiology: Update and Overview (Cham: Springer International Publishing) 1031: 641–648, doi:10.1007/978-3-319-67144-4_34, ISBN 978-3-319-67142-0, http://link.springer.com/10.1007/978-3-319-67144-4_34. Retrieved 2023-06-28
- ↑ Hurley, Caroline; Shiely, Frances; Power, Jessica; Clarke, Mike; Eustace, Joseph A.; Flanagan, Evelyn; Kearney, Patricia M. (1 November 2016). "Risk based monitoring (RBM) tools for clinical trials: A systematic review" (in en). Contemporary Clinical Trials 51: 15–27. doi:10.1016/j.cct.2016.09.003. https://linkinghub.elsevier.com/retrieve/pii/S1551714416302877.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation, grammar, and punctuation. In some cases important information was missing from the references, and that information was added.