Difference between revisions of "Template:Article of the week"
From LIMSWiki
Jump to navigationJump to searchShawndouglas (talk | contribs) m (Internal link) |
Shawndouglas (talk | contribs) (Updated article of the week text) |
||
| (390 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
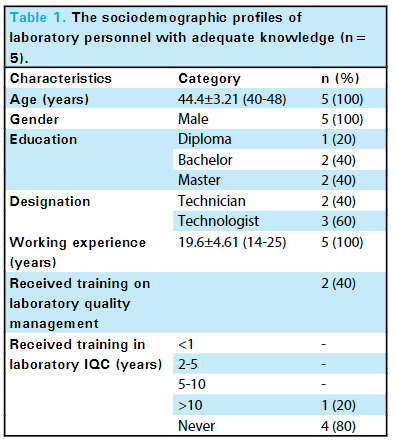
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Mishra JofNepMedAss23 61-258.png|220px]]</div> | ||
'''"[[Journal: | '''"[[Journal:Knowledge of internal quality control for laboratory tests among laboratory personnel working in a biochemistry department of a tertiary care center: A descriptive cross-sectional study|Knowledge of internal quality control for laboratory tests among laboratory personnel working in a biochemistry department of a tertiary care center: A descriptive cross-sectional study]]"''' | ||
The [[clinical laboratory]] holds a central position in patient care, and as such, ensuring accurate [[laboratory]] test results is a necessity. Internal [[quality control]] (QC) ensures day-to-day laboratory consistency. However, unless practiced, the success of laboratory [[quality management system]]s (QMSs) cannot be achieved. This depends on the efforts and commitment of laboratory personnel for its implementation. Hence, the aim of this study was to find out the knowledge of internal QC for laboratory tests among laboratory personnel working in the Department of Biochemistry, B.P. Koirala Institute of Health Sciences (BPKIHS), a tertiary care center ... ('''[[Journal:Knowledge of internal quality control for laboratory tests among laboratory personnel working in a biochemistry department of a tertiary care center: A descriptive cross-sectional study|Full article...]]''')<br /> | |||
''Recently featured'': | |||
{{flowlist | | |||
* [[Journal:Sigma metrics as a valuable tool for effective analytical performance and quality control planning in the clinical laboratory: A retrospective study|Sigma metrics as a valuable tool for effective analytical performance and quality control planning in the clinical laboratory: A retrospective study]] | |||
<br /> | * [[Journal:Why do we need food systems informatics? Introduction to this special collection on smart and connected regional food systems|Why do we need food systems informatics? Introduction to this special collection on smart and connected regional food systems]] | ||
''Recently featured'': [[Journal: | * [[Journal:Data management challenges for artificial intelligence in plant and agricultural research|Data management challenges for artificial intelligence in plant and agricultural research]] | ||
}} | |||
Revision as of 18:14, 6 May 2024
The clinical laboratory holds a central position in patient care, and as such, ensuring accurate laboratory test results is a necessity. Internal quality control (QC) ensures day-to-day laboratory consistency. However, unless practiced, the success of laboratory quality management systems (QMSs) cannot be achieved. This depends on the efforts and commitment of laboratory personnel for its implementation. Hence, the aim of this study was to find out the knowledge of internal QC for laboratory tests among laboratory personnel working in the Department of Biochemistry, B.P. Koirala Institute of Health Sciences (BPKIHS), a tertiary care center ... (Full article...)
Recently featured:
- Sigma metrics as a valuable tool for effective analytical performance and quality control planning in the clinical laboratory: A retrospective study
- Why do we need food systems informatics? Introduction to this special collection on smart and connected regional food systems
- Data management challenges for artificial intelligence in plant and agricultural research