Difference between revisions of "Template:Latest news"
Shawndouglas (talk | contribs) (Updated news.) |
|||
| (10 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
<h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">November 20, 2023:</h2> | |||
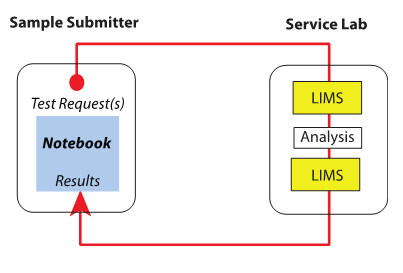
<h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;"> | [[File:Fig3 Liscouski SciStudGuideLabInfo23.png|left|180px]] '''Are you studying some sort of [[laboratory]]-based science in university?''' How well do your classes address [[laboratory informatics]] topics, particularly in the scope of industrial labs and how they operate outside of academia? If you find the discussion lacking, then his guide by industry veteran Joe Liscouski will be worth a look. In his guide ''[[LII:A Science Student's Guide to Laboratory Informatics|A Science Student's Guide to Laboratory Informatics]]'', Liscouski presents "an annotated map of the laboratory portion of a technological world, identifying critical points of interest and how they relate to one another, while making recommendations for the reader to learn more." Hope you find it useful! [[User:Shawndouglas|Shawn Douglas]] ([[User talk:Shawndouglas|talk]]) 18:48, 20 November 2023 (UTC) | ||
[[File: | |||
[[User:Shawndouglas|Shawn Douglas]] ([[User talk:Shawndouglas|talk]]) | |||
<br /> <br /> | <br /> <br /> | ||
<h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;"> | <h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">July 26, 2023:</h2> | ||
[[File: | [[File:Man and Woman Shaking Hands.jpg|left|180px]] '''Do you feel your lab needs [[laboratory informatics]] software but you're not sure how to justify it to management?''' Then [[LII:Justifying_LIMS_Acquisition_and_Deployment_within_Your_Organization|this new guide]] from Joe Liscouski and Shawn Douglas may be just what you need. Justification for a [[laboratory information management system]] (LIMS) or some other lab management solution isn't always straightforward with upper management and critical stakeholders; the process will need to be succinct and relevant, based on organizational goals, economic concerns, and practical realities. This guide will help you—whether you are a lab manager, lab technician, or someone else in the organization with a stake in seeing [[laboratory]] operations improve—understand what a LIMS is, what the alternatives are, what and why acquiring one looks like, and what needs to be considered in putting together a competent and persuasive LIMS project proposal. This guide also includes a handy Microsoft Excel workbook that will help act as a "cheat sheet" for persuading stakeholders to better buy into your vision of LIMS acquisition and deployment within your organization. Enjoy! [[User:Shawndouglas|Shawn Douglas]] ([[User talk:Shawndouglas|talk]]) 16:32, 26 July 2023 (UTC) | ||
[[User:Shawndouglas|Shawn Douglas]] ([[User talk:Shawndouglas|talk]]) | |||
<br /> | <br /> | ||
Latest revision as of 16:37, 19 February 2024
November 20, 2023:
Are you studying some sort of laboratory-based science in university? How well do your classes address laboratory informatics topics, particularly in the scope of industrial labs and how they operate outside of academia? If you find the discussion lacking, then his guide by industry veteran Joe Liscouski will be worth a look. In his guide A Science Student's Guide to Laboratory Informatics, Liscouski presents "an annotated map of the laboratory portion of a technological world, identifying critical points of interest and how they relate to one another, while making recommendations for the reader to learn more." Hope you find it useful! Shawn Douglas (talk) 18:48, 20 November 2023 (UTC)
July 26, 2023:
Do you feel your lab needs laboratory informatics software but you're not sure how to justify it to management? Then this new guide from Joe Liscouski and Shawn Douglas may be just what you need. Justification for a laboratory information management system (LIMS) or some other lab management solution isn't always straightforward with upper management and critical stakeholders; the process will need to be succinct and relevant, based on organizational goals, economic concerns, and practical realities. This guide will help you—whether you are a lab manager, lab technician, or someone else in the organization with a stake in seeing laboratory operations improve—understand what a LIMS is, what the alternatives are, what and why acquiring one looks like, and what needs to be considered in putting together a competent and persuasive LIMS project proposal. This guide also includes a handy Microsoft Excel workbook that will help act as a "cheat sheet" for persuading stakeholders to better buy into your vision of LIMS acquisition and deployment within your organization. Enjoy! Shawn Douglas (talk) 16:32, 26 July 2023 (UTC)