Journal:Precision nutrition: Maintaining scientific integrity while realizing market potential
| Full article title | Precision nutrition: Maintaining scientific integrity while realizing market potential |
|---|---|
| Journal | Frontiers in Nutrition |
| Author(s) | Berciano, Silvia; Figueiredo, Juliana; Brisbois, Tristin D.; Alford, Susan; Koecher, Katie; Eckhouse, Sara; Ciati, Roberto; Kussmann, Martin; Ordovas, Jose M.; Stebbins, Katie; Blumberg, Jeffrey B. |
| Author affiliation(s) | Tufts University, PepsiCo, Novo Nordisk, General Mills, FoodShot Global, Barilla G&R, German Entrepreneurship |
| Primary contact | jeffrey dot blumberg at tufts dot edu |
| Editors | Zhu, Zhenjun |
| Year published | 2022 |
| Volume and issue | 9 |
| Article # | 979665 |
| DOI | 10.3389/fnut.2022.979665 |
| ISSN | 2296-861X |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.frontiersin.org/articles/10.3389/fnut.2022.979665/full |
| Download | https://www.frontiersin.org/articles/10.3389/fnut.2022.979665/pdf (PDF) |
Abstract
Precision nutrition (PN) is an approach to developing comprehensive and dynamic nutritional recommendations based on individual variables, including genetics, microbiome, metabolic profile, health status, physical activity, dietary pattern, and food environment, as well as socioeconomic and psychosocial characteristics. PN can help answer the question “what should I eat to be healthy?”, recognizing that what is healthful for one individual may not be the same for another, and understanding that health and responses to diet change over time. The growth of the PN market has been driven by increasing consumer interest in individualized products and services coupled with advances in technology, analytics, and omic sciences. However, important concerns are evident regarding the adequacy of scientific substantiation supporting claims for current products and services. An additional limitation to accessing PN is the current cost of diagnostic tests and wearable devices. Despite these challenges, PN holds great promise as a tool to improve "healthspan" and reduce healthcare costs. Accelerating advancement in PN will require: (a) investment in multidisciplinary collaborations to enable the development of user-friendly tools applying technological advances in omics, sensors, artificial intelligence (AI), big data management, and analytics; (b) engagement of healthcare professionals and payers to support equitable and broader adoption of PN as medicine shifts toward preventive and personalized approaches; and (c) system-wide collaboration between stakeholders to advocate for continued support for evidence-based PN, develop a regulatory framework to maintain consumer trust and engagement, and allow PN to reach its full potential.
Keywords: precision nutrition, personalized nutrition, omics, genetics, microbiome, metabolic health, wearable devices
Introduction
Nutrition is a fundamental pillar of health, and diet is the modifiable factor that exerts the greatest impact on human health and wellbeing.[1] Dietary recommendations have traditionally been based on a one-size-fits-all approach that assumes individual nutritional requirements and responses mimic the average response observed in study populations.[2] The advancement of personalized nutrition or precision nutrition (PN) strategies has improved our understanding of how factors such as genetics, microbiome, and metabolic profiles may predict whether what we eat supports or harms our health and to what degree.[3]
Studies in the field of nutritional genomics have unveiled associations between genetic factors and metabolic responses to food, nutrient requirements, dietary preferences, and disease outcomes.[4][5][6][7] Advances in this and other areas of PN have added new dimensions that help explain the variability in responses observed in otherwise well-controlled trials of diet and nutrients.[8] In particular, promising research results support the predictive potential of assessments of the gut microbiome and metabolome, among other factors, and showcase the individual but malleable qualities of our biology.[9][10]
As we bring new perspectives to the multiple dimensions of food and health, we are also overcoming some of the barriers created by previous reductionist thinking about nutrition. It is in this context that PN is driving the scientific journey toward a more personalized, predictive, and integrative systems approach to understanding how nature and nurture interact to shape our health and wellbeing.
The precision nutrition approach: One size does not fit all
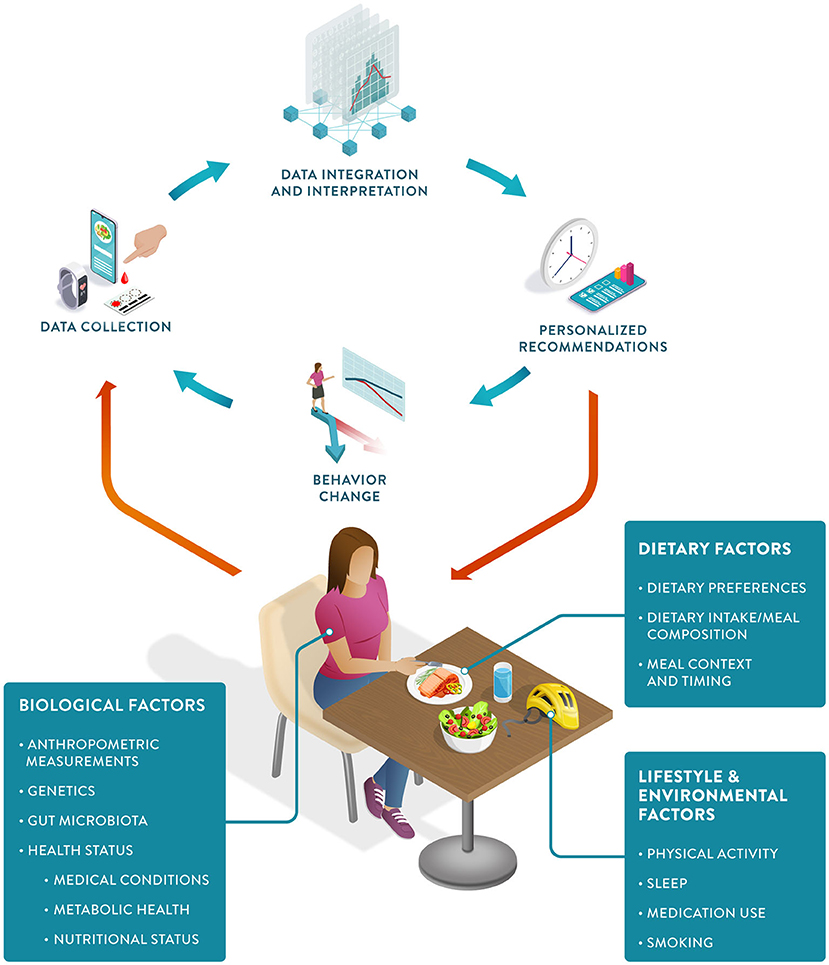
PN can be defined as an approach that uses individual data to predict how a person will respond to specific foods or dietary patterns and tailors dietary recommendations to their individual needs. These personalized recommendations are expected to elicit behavioral changes that would lead to improvements in the health trajectory of the person (Figure 1).
|
PN represents an advancement from both traditional dietary advice and earlier personalized approaches.[11] Despite recognized causal links between diet and health outcomes, traditional dietary intervention strategies to reduce the burden of chronic diseases have had a limited impact.[5] This is due to a combination of poor adherence to dietary recommendations and different individual responses to the same food and dose.[12][13][14] PN has the potential to tackle both challenges. Personalized recommendations have been shown to increase compliance, and predictions of the direction and magnitude of an individual's response to food allow for the development of more effective recommendations tailored to their specific needs and metabolism.[15]
Aided by a wealth of data now gathered via wearable devices, smartphones, and diagnostic tests, PN holds the promise of a more cost-effective approach to health promotion.[16][17] These data can be analyzed and integrated using computational methods to generate both qualitative and quantitative “just-in-time” nutritional recommendations for the individual client or patient.
As PN continues to advance, it has the potential to further enhance our understanding and application of nutrition and significantly impact outcomes such as health maintenance, resilience and restoration, fertility, physical capacity, and cognitive performance. Key attributes of the PN approach are listed in Table 1.
| ||||||||||||||||||
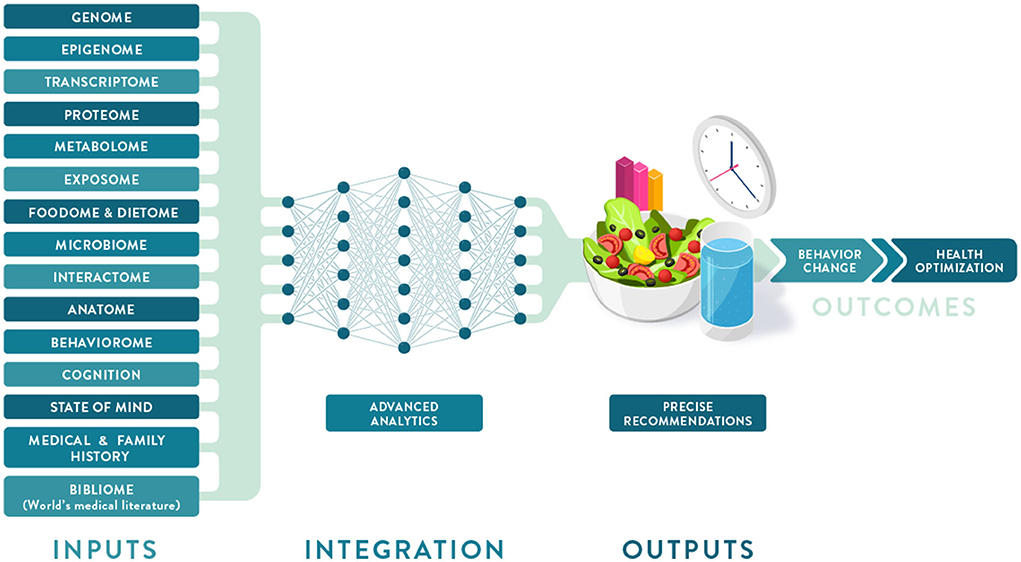
Individual variation in dietary behaviors and responses has been shown to be multi-faceted and includes genetics, metabolic profiling, and meta-omic signatures (such as metagenomics and meta-transcriptomics), as well as psychological, anthropometric, sociodemographic, and environmental factors (Figure 2). To condense some of these layers of information into more manageable inputs, many of these predictors are presented as “-types” or signatures. While “genotype” generally refers to a single locus in our genome, the terms "metabotype," "nutritype," "ageotype," and "phenotype" refer to composite measures that combine a large number of variables related to our metabolism, diet, aging, and other traits.
|
It is clear that the foundation of PN has been built largely on evidence from omics studies that are more focused on molecular biology and nutritional biochemistry than environmental and social drivers of eating behavior. However, considering additional characteristics—including sensorial responses, personal circumstances, values, attitudes, behaviors, and social determinants of health (SDOH)—will facilitate the development of PN solutions that are adequately tailored to, accepted by, and adopted by the individual, resulting in improved lifestyles and lasting health.
Big data and analytics in precision nutrition
Advances in data acquisition and analytics have enabled omics to build connections between large data sets to further understand the multiple facets of food intake behavior and nutrient metabolism. Technological advances in omics sciences, artificial intelligence (AI), and sensors (especially wearable devices) also have the potential to revolutionize how nutrition research is conducted and how dietary insights are presented to and used by the public.
Traditional dietary assessment methods such as food frequency questionnaires, diet records, and recalls have limited resolution to provide a precise intake profile and can be burdensome to complete. The development of mobile apps offering image recognition to quantify meals and wearable sensors to detect and capture nutrient intake, along with barcode scanners to facilitate the recognition of packaged foods, may result in more precise, real-time, and user-friendly dietary assessments.[18]
The growing presence of sensors and personal electronic devices in homes will complement and increase the accuracy of image- and questionnaire-based methods to track dietary intake. Next-generation wearables will be able to continuously and non-invasively monitor blood glucose and other biomarkers before “lab-on-a-chip” implants are created, which will combine sensing capabilities with delivery systems. Smart appliances and toilets will collect data on food intake, nutrient status, dietary responses, and health biomarkers of each consenting member of the household.[19] Smart pills are already considered an inexpensive tool for direct sampling of microbial communities in the gastrointestinal tract. They can provide new insights into the role of diet in mediating host-microbe interactions and metabolism.[20][21] At-home sampling and testing using fecal collection kits, dried blood spot cards, and continuous glucose monitors are now commercially available. More comprehensive devices under development may replace some of the current options and enable higher-resolution and real-time nutrient, behavior, and health tracking.
Multiomic profiling plays a major role in research directed at identifying sets of biomarkers relevant to health maintenance and disease prevention. While the advent of ultra-connected devices and the internet of things (IoT) can revolutionize nutrition and health data collection, AI has already changed how big data are analyzed and interpreted.[22] AI is instrumental in the analysis of massive real-world data collected using wearables or diagnostic tools to better detect patterns and predict health trajectories. Common applications of machine learning (ML) algorithms in nutrition include the discovery and validation of new bioactive ingredients, integration of dietary and health data, development of predictive models, and recommendations to optimize health outcomes.
PN tools available to consumers—many of whom are early adopters of this technology and eager to share their insights and data—are becoming more numerous and accessible, fueling interest in new study designs in personal and citizen science. These approaches may reduce the time an individual spends on an intervention before a positive or adverse event is detected.[23] Importantly, trials directed to PN can reveal subclinical departures from health to disease, thereby enabling the discovery of early markers of deviation from a healthy trajectory that inform disease prevention and health maintenance.[24]
Developing personalized nutrition products and services
The translation of PN science into products and services can be enhanced by considering the balance of benefits and risks for both consumers and patients. Benefits may include the improvement of a specific health outcome, the convenience of user-friendly digital tools, and the efficacy of a more personalized approach. On the other hand, risks may result from the high cost of repeated omics testing, the time burden for users due to complex programs, and unmet expectations where gaps exist between the science and product claims. Risks also include concerns regarding trust, privacy, and control of data.
Several privately and publicly funded large-scale studies are underway to gather key data and develop the necessary knowledge and methods to elucidate which metrics are most important, what degree of granularity or resolution is necessary, and which signatures of health and disease should receive priority for testing.[3][25] These findings are expected to promote innovation with validated and novel PN products and services in the healthcare and food industries. In addition, open-source tools are being developed to support individuals and their healthcare providers, offering more personalized dietary and lifestyle recommendations to complement population-based recommendations such as the Dietary Guidelines for Americans (DGA).[2]
Some issues of concern regarding the development of commercial PN products and services include insufficient scientific evidence supporting effectiveness, the limited predictive power of the underlying algorithms, and unsubstantiated claims. The majority of available commercial PN products and programs are collecting data and refining algorithms as they are being used. This progressive generation of data and knowledge could be at the expense of the consumer if the interpretations or recommendations being generated are incorrect or ineffective, e.g., when they suggest causality without evidence. Even when there are statistically significant associations between specific factors and health-related outcomes, several commercial solutions appear to lack validation, sufficient predictive power, clinical relevance, and/or actionable advice. The level of phenotyping and comprehensive predictive analytics found in some advanced research settings do not seem feasible for direct commercial applications at this time.
The PN market is largely unregulated and dominated by small companies. Even wellbeing solutions that are generally considered to pose little threat to the individual carry a risk of misleading consumers if such products or services are not rigorously designed and their benefits clearly evaluated and communicated. The growing demand for PN tests, personalized diets, and supplements in the absence of a reasonable regulatory framework could lead to an erosion of consumer trust. The development and adoption of evidence-based PN solutions ultimately depend on consumers sharing personal data, so credibility, transparency, and trust are essential to the responsible growth of this industry.
Future efforts in PN are expected to involve the use of key biomarker panels and integrated analyses. Separate layers of data can be collected by different partners, but agreed-upon standards are needed to facilitate merging datasets so that a more accurate interpretation can be achieved. PN solutions can also be tailored to well-defined consumer or patient groups which encompass individuals with similar needs. Scientific rigor, relevance of diet/health predictors, convenience, and access are essential considerations in the development and democratization of PN. Making these attributes a priority will accelerate innovation in PN and its integration into healthcare strategies for individualized disease prevention, treatment, and care.
Precision nutrition and the future of healthcare
Human life expectancy has increased by three decades over the last century. However, this lifespan extension has not been matched by an improvement in "healthspan," i.e., years lived without disease.[26] Suboptimal diets are responsible for one in five adult deaths globally.[27] The epidemic of diet-related chronic conditions accounts for 90% of the $3.5 trillion in annual health care expenditures in the US.[28]
However, the impact of poor diet on human health has not been fully recognized in healthcare. Public health approaches have addressed this burden from either the perspective of hunger and malnutrition (including food and nutrition insecurity) or that of overweight and obesity (focused on promotion of dietary quality and reduction of energy density).[29] Metabolic health is a current metric of interest and defined based on a combination of risk factors: fasting glucose (<100 mg/dL), hemoglobin A1c (<5.7%), blood pressure (systolic <120 and diastolic <80 mmHg), triglycerides (<150 mg/dL), high-density lipoprotein cholesterol (≥40/50 mg/dL for men/women), anthropometrics (waist circumference <102/88 cm for men/women), and not taking any related medication.[30] Only 12% of adults in the US are metabolically healthy, including less than one-third of normal-weight adults.[27] PN can facilitate metabolic health assessment by using more sensitive markers, enabling earlier detection of metabolic dysregulation and providing more effective dietary strategies to regain metabolic health.
Deep phenotyping can provide a better understanding of individual risk factors, dietary responses, and metabolic regulation. Paired with early detection and correction of health trajectories, deep phenotyping holds a promise for increasing our healthspan. Digital twins are high-resolution models of a deeply phenotyped subject that can be computationally subjected to unlimited different nutritional interventions.[31] Though the cost and complexity of digital twins in precision medicine have been compared to the Human Genome Project, digital twin approaches may be useful in simulating individual dietary effects and generating recommendations for health optimization.[32] This concept represents a very high level of personalization, standing in contrast to current approaches that rely on stratification to more simply distinguish or cluster individuals with similar needs or responses. Unlocking the full potential of such big data approaches exceeds the capability of existing computers. However, it may be feasible in five to 10 years when hybrid machines that combine quantum and conventional processing become essential tools in the analysis of highly complex datasets involving multiple interacting variables.
Individualized or n-of-1 trials are one-person trials where the number of measured variables and the sampling frequency can capture the intra-individual variability across a health trajectory, enabling comparisons between different interventions and empirical determination of optimal diet for a specific person.[33] Although such findings are not expected to be generalizable, they are compatible with the goal of clinical practice: the care of individual patients.[23] The n-of-1 study design represents a promising type of PN research, particularly when multiple n-of-1 studies are conducted in a coordinated manner and can be aggregated to identify subgroup effects. Aggregated coordinated n-of-1 studies cluster individuals with similar characteristics or responses (e.g., responders vs. non-responders), can help achieve greater resolution in nutrition science, and expand the research approaches used to substantiate PN programs. Therefore, they present excellent opportunities to advance this field.
Non-invasive wearable- and app-enhanced measurements of health and lifestyle parameters such as blood pressure, blood glucose, body composition, dietary intake, and physical activity are key enablers of consumer empowerment. Their proactive use in healthcare can promote a shift to measurable prevention and healthier habits, compared to the current reactive approach in which healthcare is sought primarily when health declines. The “quantified self” trend refers to wearable-based and app-enabled self-measurements of health parameters by end-users, consumers, and patients.[34] This is a key driver for the adoption of innovative PN solutions, as consumers can autonomously measure the benefits of dietary and lifestyle changes. However, the real value is created when these personal data are put into context: apps and tools need to be linked to credible databases to generate sound, actionable, and personalized advice.
Early adopters of PN are generally individuals driving their own health journey, often without expert advice or financial incentives. They tend to be younger, healthier, more affluent, and possess higher nutrition and health literacy.[35] While this group can benefit from PN programs, connecting consumers and patients whose need for PN assessment and recommendations is more urgent should be a priority. Healthcare systems can be instrumental in fostering broader adoption of PN by emphasizing its role in health promotion and disease prevention and shifting its principal focus from a disease-centric model. This approach will require the education of patients and healthcare providers, many of whom are not knowledgeable about how diet can drive health outcomes.[36]
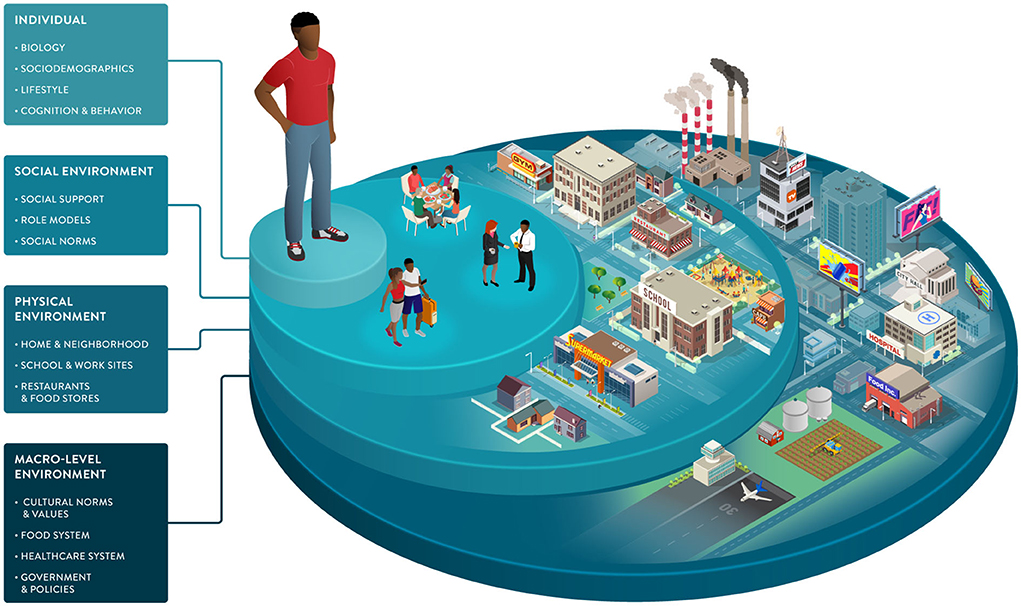
Environmental factors and SDOH impact food choices and the opportunities and barriers that support or hinder healthy behaviors. As part of the broader environment, macro-level factors play an indirect but powerful role in driving individual health. This dimension includes healthcare systems, public health policies, and food systems engaging in activities from harvesting and processing to commercialization and marketing (Figure 3).
|
Advancing PN requires the development of precise measures of exposures, behaviors, and susceptibilities in diverse populations. Shifting the focus from treatment to prevention and delivering the right intervention to the right population at the right time can be achieved with precision public health approaches that complement individual-focused PN strategies.[37]
This path to integrated healthcare is not short: moving from reactive treatment to proactive prevention approaches will require time, investment, training, and clear guidelines but is expected to generate significant value. In a report from the McKinsey Company[38], it is estimated that the effective use of big data by the US healthcare sector would create $300 billion in value every year. Most of this estimated value would be derived from the identification of the most clinically effective and cost-effective treatments based on data already being generated by health care providers.
Insurers will also play a crucial role in PN adoption, providing incentives when clients engage with PN measures. Healthcare systems can reduce costs when pharmacological treatments are replaced by or combined with less expensive nutritional solutions, especially for chronic and lifestyle-related conditions that cannot be sustainably managed by pharmaceutical means alone.[39] However, PN approaches must first show evidence of clinical efficacy and cost-effectiveness. Robust returns on investment (ROI) in the form of improvements in clinical outcomes, patient and provider experience, and lower healthcare costs would justify reimbursement. In addition to healthcare systems, PN could also serve as an integral part of workplace wellness programs, encouraging employees to be proactive about health maintenance and deploying evidence-based health apps that provide actionable lifestyle recommendations.[40]
Best practices and standards in precision nutrition
Nutrition regulators will need to develop policies regarding evidence generation, approval, and reimbursement of PN solutions. This applies to approaches delivered in healthcare settings as well as direct-to-consumer products and services. Given the fast pace of research and innovation in this field, it is critical for academia, industry, and policymakers to work together and generate effective regulatory frameworks and guidelines, starting with industry standards and best practices.
The need for and growing interest in PN across different organizations and industries calls for a joint effort to establish a consensus framework covering definitions, science, commercialization, and communication. Innovation will move faster and more effectively if a common language between academia, industry, and regulatory bodies is established. Standards are the basis for mutual understanding of PN products and services, and for improving transparency and acceptance of this approach as a strategy in healthcare and a solution for consumers. Moreover, best practices in PN science, commercialization, and communication are key to enabling the replication and comparison of findings and fostering trust and credibility with consumers, patients, and healthcare providers.
Standards will also apply to novel study designs and analytical approaches, which are necessary to overcome experimental limitations and establish effective PN science. Although the n-of-1 approach is not novel, it has gained traction over the last decade.[41] Such designs require additional methodological and statistical considerations to ensure proper data analysis and interpretation to generate sensible personalized advice.[42]
Currently, the lack of diversity in PN research, as in many research areas, is a limitation, as it could lead to inappropriate application of PN algorithms and impede equitable implementation. The National Institutes of Health (NIH) Precision Nutrition initiative addresses this issue by leveraging the "All of Us" cohort. Such diverse and inclusive populations are crucial to minimizing information gaps in PN trials, from ethnicity to environmental exposures to varied SDOH. Closing this gap, with an emphasis on underrepresented populations, will help make effective and personalized dietary approaches possible for all in the near future.[35]
Developing and implementing research best practices is key to conducting high-quality science and generating the robust evidence foundation needed to substantiate PN products, services, and solutions. This is important as most medical and nutrition research is funded by industry, not by public sources.[43] PN solutions should provide evidence of specific health benefits or amelioration of health symptoms or pathology. Rising consumer demand drives PN companies to constantly reassess their markets and innovate rapidly, launching new products in a field that does not yet have a strong evidence foundation. Caution is warranted in putting marketing ahead of science. In moving forward to establish PN practices, there are also legal challenges such as ensuring privacy and ethical use of data when collecting and processing personal information.[44]
Currently, there is no specific framework for evaluating PN solutions. Potential regulatory approaches include federal regulation and industry self-regulation. While it is clear that industry standards and federal policies can complement each other, strengths and limitations specific to each regulatory approach are listed in Table 2. Academics, industry leaders, and policymakers must work together to establish scientific standards to define which claims should be allowed and how to substantiate them. That set of common rules, in turn, must be translated into regulatory frameworks.
| ||||||||
While government regulation and enforcement guidelines for PN appear inevitable, they will need to be prioritized and funded and will take years to create. A proactive consortium of stakeholders may help establish a consensus framework for the definition, science, practice, and communication of PN before developing a broader regulatory framework.
Advocacy and communication strategies for precision nutrition
Effective communication of PN is key to promoting trust and the adoption of evidence-based solutions. The novelty and inherent complexity of PN, combined with the legacy of nutrition controversies and misinformation, contribute to the confusion that consumers, healthcare providers, and regulators face when encountering, interpreting, and formulating nutrition messages.
Growing access to information continues to transform the way people think about nutrition and its connections to health. There are several channels through which the general public can access nutrition information; some reflect general recommendations (e.g., DGA), while others provide a biased view that creates opportunities for nutrition misinformation.[45] The lack of nutrition and health literacy is a major challenge, and nutrition communications outside of trustworthy sources are fragmented, especially on social media.[46]
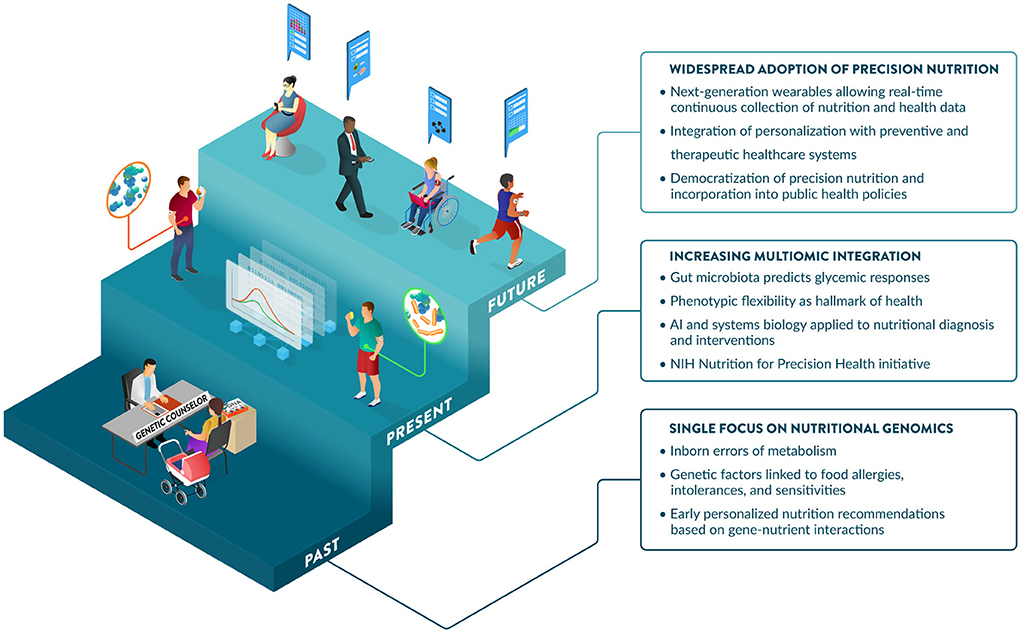
Communications regarding the science of PN often portray different degrees of excitement about this field, from overly optimistic to openly obstructive or visibly skeptical.[47] Support from the NIH has significantly increased the profile of the PN field. This includes the NIH Nutrition for Precision Health Initiative[48], which has a strong focus on PN in their 2020–2030 strategic plan[25], and a notable investment of $170 million for a new program to develop algorithms to predict individual responses to food and dietary routines.[49] PN is maturing and expected to play a major role in disease prevention and health promotion over the next decade. Further, perceptions of benefits, costs, risks, and uncertainties associated with PN influence consumer attitudes and acceptance.[50] Promoting nutrition and health literacy and developing best practices and standards for the effective communication of PN to different audiences will be critical to enabling its adoption. Guidance from professional societies and government agencies on responsible communications regarding the application of PN today and its promise for the future would help address this challenge. Significant advances and investments in large PN studies are generating the data, methods, and knowledge to enable the incorporation of PN into healthcare strategies as early as 2030.[48] Key developments and hallmarks of the early days, the present, and the foreseeable future of PN are illustrated in Figure 4.
|
Conclusions and recommendations
PN is a data-driven approach to assessing health that tailors dietary recommendations to individual needs. When fully embedded in the healthcare system, PN should have an impact on both personal and public health. Advancing the science and the adoption of PN will require a significant investment in multidisciplinary collaborations that translate the fast-moving technological advances in omics, sensors, AI, and big data management and analytics into powerful and user-friendly tools. To allow PN to reach its full potential while maintaining consumer trust and engagement, regulatory frameworks from the industry and/or government will need to be established, along with guidance on relevant ethical and legal aspects of the practice. Healthcare professionals and providers will need to be trained to provide PN services, supporting a broad and equitable adoption of PN. Collaboration between stakeholders in the world of nutrition and health will be required to expand the scientific and clinical evidence of the efficacy and effectiveness of PN and ensure that system-wide barriers to access and coverage can be overcome.
Abbreviations, acronyms, and initialisms
- AI: artificial intelligence
- DGA: Dietary Guidelines for Americans
- IoT: internet of things
- ML: machine learning
- NIH: National Institutes of Health
- PN: precision nutrition
- ROI: return on investment
- SDOH: social determinants of health
Acknowledgements
The authors thank illustrator David Cunha (Ideatomik) for designing the figures and Dr. Keith Grimaldi (DNAfit) for useful conversation on PN regulation and best practices.
Author contributions
KS and SB conceived the idea for this perspective. SA, TB, RC, SE, KK, MK, JO, and JB provided input to inform the review outline and draft. SB and JF drafted the manuscript. All authors contributed to reviewing, editing, and approving the final version of the manuscript.
Funding
This work was supported by the Tufts Food & Nutrition Innovation Council (FNIC). The FNIC Precision Nutrition Working Group is a collaboration between the Food & Nutrition Innovation Institute in the Friedman School of Nutrition Science and Policy at Tufts University and members of its Council, a multi-stakeholder coalition working to rethink the global food system with a focus on health, equity, and sustainability.
Conflict of interest
Author TB is employed by PepsiCo Inc. and co-chair of the FNIC Precision Nutrition Working Group. Author SA is an employee of Novo Nordisk Inc. and is a stockholder and a co-chair of the FNIC Precision Nutrition Working Group. Author KK is an employee of General Mills, Inc. Author SE is an employee of FoodShot Global. Author RC is an employee of Barilla G&R. Author MK is affiliated with German Entrepreneurship, USA. Author JO serves on scientific advisory boards for Nutrigenomix, Zoe Global, GNC, PepsiCo, and Weight Watchers. Author JB serves on scientific advisory boards for Segterra, Inc. (Inside Tracker) and January.ai, Inc. (outside of the submitted work).
The remaining authors declare that the work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- ↑ Ordovas, Jose M.; Corella, Dolores (22 September 2004). "NUTRITIONAL GENOMICS" (in en). Annual Review of Genomics and Human Genetics 5 (1): 71–118. doi:10.1146/annurev.genom.5.061903.180008. ISSN 1527-8204. https://www.annualreviews.org/doi/10.1146/annurev.genom.5.061903.180008.
- ↑ 2.0 2.1 U.S. Department of Agriculture and U.S. Department of Health and Human Services (December 2020). "Dietary Guidelines for Americans 2020–2025". https://www.dietaryguidelines.gov/. Retrieved 09 June 20222.
- ↑ 3.0 3.1 Berry, Sarah E.; Valdes, Ana M.; Drew, David A.; Asnicar, Francesco; Mazidi, Mohsen; Wolf, Jonathan; Capdevila, Joan; Hadjigeorgiou, George et al. (1 June 2020). "Human postprandial responses to food and potential for precision nutrition" (in en). Nature Medicine 26 (6): 964–973. doi:10.1038/s41591-020-0934-0. ISSN 1078-8956. PMC PMC8265154. PMID 32528151. https://www.nature.com/articles/s41591-020-0934-0.
- ↑ Lopez-Miranda, José; Williams, Christine; Lairon, Denis (1 September 2007). "Dietary, physiological, genetic and pathological influences on postprandial lipid metabolism" (in en). British Journal of Nutrition 98 (3): 458–473. doi:10.1017/S000711450774268X. ISSN 0007-1145. https://www.cambridge.org/core/product/identifier/S000711450774268X/type/journal_article.
- ↑ 5.0 5.1 Ordovas, Jose M; Ferguson, Lynnette R; Tai, E Shyong; Mathers, John C (13 June 2018). "Personalised nutrition and health" (in en). BMJ: bmj.k2173. doi:10.1136/bmj.k2173. ISSN 0959-8138. PMC PMC6081996. PMID 29898881. https://www.bmj.com/lookup/doi/10.1136/bmj.k2173.
- ↑ Berciano, Silvia; Lai, Chao‐Qiang; Herranz, Jesus; Aslibekyan, Stella; Claas, Steve A; Irvin, Marguerite R; Tsai, Michael Y; Hopkins, Paul N et al. (1 April 2017). "Behavior related genes, dietary preferences and anthropometric traits" (in en). The FASEB Journal 31 (S1). doi:10.1096/fasebj.31.1_supplement.299.1. ISSN 0892-6638. https://onlinelibrary.wiley.com/doi/10.1096/fasebj.31.1_supplement.299.1.
- ↑ Perez-Martinez, Pablo; Phillips, Catherine; Delgado-Lista, Javier; Garcia-Rios, Antonio; Lopez-Miranda, Jose; Perez-Jimenez, Francisco (31 January 2014). "Nutrigenetics, Metabolic Syndrome Risk and Personalized Nutrition" (in en). Current Vascular Pharmacology 11 (6): 946–953. doi:10.2174/157016111106140128120911. http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1570-1611&volume=11&issue=6&spage=946.
- ↑ Rodgers, Griffin P.; Collins, Francis S. (25 August 2020). "Precision Nutrition—the Answer to “What to Eat to Stay Healthy”" (in en). JAMA 324 (8): 735. doi:10.1001/jama.2020.13601. ISSN 0098-7484. https://jamanetwork.com/journals/jama/fullarticle/2769420.
- ↑ Zeevi, David; Korem, Tal; Zmora, Niv; Israeli, David; Rothschild, Daphna; Weinberger, Adina; Ben-Yacov, Orly; Lador, Dar et al. (1 November 2015). "Personalized Nutrition by Prediction of Glycemic Responses" (in en). Cell 163 (5): 1079–1094. doi:10.1016/j.cell.2015.11.001. https://linkinghub.elsevier.com/retrieve/pii/S0092867415014816.
- ↑ Fiamoncini, Jarlei; Rundle, Milena; Gibbons, Helena; Thomas, E. Louise; Geillinger‐Kästle, Kerstin; Bunzel, Diana; Trezzi, Jean‐Pierre; Kiselova‐Kaneva, Yoana et al. (1 October 2018). "Plasma metabolome analysis identifies distinct human metabotypes in the postprandial state with different susceptibility to weight loss‐mediated metabolic improvements" (in en). The FASEB Journal 32 (10): 5447–5458. doi:10.1096/fj.201800330R. ISSN 0892-6638. https://onlinelibrary.wiley.com/doi/abs/10.1096/fj.201800330R.
- ↑ Bush, Corinne L.; Blumberg, Jeffrey B.; El-Sohemy, Ahmed; Minich, Deanna M.; Ordovás, Jóse M.; Reed, Dana G.; Behm, Victoria A. Yunez (2 January 2020). "Toward the Definition of Personalized Nutrition: A Proposal by The American Nutrition Association" (in en). Journal of the American College of Nutrition 39 (1): 5–15. doi:10.1080/07315724.2019.1685332. ISSN 0731-5724. https://www.tandfonline.com/doi/full/10.1080/07315724.2019.1685332.
- ↑ Martin, Kathleen A; Bowen, Deborah J; Dunbar-Jacob, Jacqueline; Perri, Michael G (1 October 2000). "Who Will Adhere? Key Issues in the Study and Prediction of Adherence in Randomized Controlled Trials" (in en). Controlled Clinical Trials 21 (5): S195–S199. doi:10.1016/S0197-2456(00)00078-7. https://linkinghub.elsevier.com/retrieve/pii/S0197245600000787.
- ↑ Leme, Ana Carolina B.; Hou, Sophia; Fisberg, Regina Mara; Fisberg, Mauro; Haines, Jess (23 March 2021). "Adherence to Food-Based Dietary Guidelines: A Systemic Review of High-Income and Low- and Middle-Income Countries" (in en). Nutrients 13 (3): 1038. doi:10.3390/nu13031038. ISSN 2072-6643. PMC PMC8004702. PMID 33807053. https://www.mdpi.com/2072-6643/13/3/1038.
- ↑ Mazidi, Mohsen; Valdes, Ana M; Ordovas, Jose M; Hall, Wendy L; Pujol, Joan C; Wolf, Jonathan; Hadjigeorgiou, George; Segata, Nicola et al. (1 September 2021). "Meal-induced inflammation: postprandial insights from the Personalised REsponses to DIetary Composition Trial (PREDICT) study in 1000 participants" (in en). The American Journal of Clinical Nutrition 114 (3): 1028–1038. doi:10.1093/ajcn/nqab132. ISSN 0002-9165. PMC PMC8408875. PMID 34100082. https://academic.oup.com/ajcn/article/114/3/1028/6293856.
- ↑ Jinnette, Rachael; Narita, Ai; Manning, Byron; McNaughton, Sarah A; Mathers, John C; Livingstone, Katherine M (1 June 2021). "Does Personalized Nutrition Advice Improve Dietary Intake in Healthy Adults? A Systematic Review of Randomized Controlled Trials" (in en). Advances in Nutrition 12 (3): 657–669. doi:10.1093/advances/nmaa144. ISSN 2161-8313. PMC PMC8166555. PMID 33313795. https://academic.oup.com/advances/article/12/3/657/6031638.
- ↑ Dehghani Zahedani, Ashkan; Shariat Torbaghan, Solmaz; Rahili, Salar; Karlin, Kirill; Scilley, Darrin; Thakkar, Riya; Saberi, Maziyar; Hashemi, Noosheen et al. (1 July 2021). "Improvement in Glucose Regulation Using a Digital Tracker and Continuous Glucose Monitoring in Healthy Adults and Those with Type 2 Diabetes" (in en). Diabetes Therapy 12 (7): 1871–1886. doi:10.1007/s13300-021-01081-3. ISSN 1869-6953. PMC PMC8266934. PMID 34047962. https://link.springer.com/10.1007/s13300-021-01081-3.
- ↑ Price, James Christopher; Santos, Heitor Oliveira; Bueno, Allain Amador (1 January 2022). "The effectiveness of automated digital health solutions at successfully managing obesity and obesity-associated disorders: A PICO-structured investigation" (in en). DIGITAL HEALTH 8: 205520762210913. doi:10.1177/20552076221091351. ISSN 2055-2076. PMC PMC8990694. PMID 35401996. http://journals.sagepub.com/doi/10.1177/20552076221091351.
- ↑ Skinner, Andy; Toumpakari, Zoi; Stone, Christopher; Johnson, Laura (2 July 2020). "Future Directions for Integrative Objective Assessment of Eating Using Wearable Sensing Technology". Frontiers in Nutrition 7: 80. doi:10.3389/fnut.2020.00080. ISSN 2296-861X. PMC PMC7343846. PMID 32714939. https://www.frontiersin.org/article/10.3389/fnut.2020.00080/full.
- ↑ Park, Seung-min; Won, Daeyoun D.; Lee, Brian J.; Escobedo, Diego; Esteva, Andre; Aalipour, Amin; Ge, T. Jessie; Kim, Jung Ha et al. (6 April 2020). "A mountable toilet system for personalized health monitoring via the analysis of excreta" (in en). Nature Biomedical Engineering 4 (6): 624–635. doi:10.1038/s41551-020-0534-9. ISSN 2157-846X. PMC PMC7377213. PMID 32251391. https://www.nature.com/articles/s41551-020-0534-9.
- ↑ Waimin, Jose Fernando; Nejati, Sina; Jiang, Hongjie; Qiu, Jake; Wang, Jianghsan; Verma, Mohit S.; Rahimi, Rahim (2020). "Smart capsule for non-invasive sampling and studying of the gastrointestinal microbiome" (in en). RSC Advances 10 (28): 16313–16322. doi:10.1039/C9RA10986B. ISSN 2046-2069. PMC PMC9052936. PMID 35498852. http://xlink.rsc.org/?DOI=C9RA10986B.
- ↑ Cummins, G. (1 October 2021). "Smart pills for gastrointestinal diagnostics and therapy" (in en). Advanced Drug Delivery Reviews 177: 113931. doi:10.1016/j.addr.2021.113931. https://linkinghub.elsevier.com/retrieve/pii/S0169409X21003240.
- ↑ Xu, Yongjun; Liu, Xin; Cao, Xin; Huang, Changping; Liu, Enke; Qian, Sen; Liu, Xingchen; Wu, Yanjun et al. (1 November 2021). "Artificial intelligence: A powerful paradigm for scientific research" (in en). The Innovation 2 (4): 100179. doi:10.1016/j.xinn.2021.100179. PMC PMC8633405. PMID 34877560. https://linkinghub.elsevier.com/retrieve/pii/S2666675821001041.
- ↑ 23.0 23.1 Lillie, Elizabeth O; Patay, Bradley; Diamant, Joel; Issell, Brian; Topol, Eric J; Schork, Nicholas J (1 March 2011). "The n-of-1 clinical trial: the ultimate strategy for individualizing medicine?" (in en). Personalized Medicine 8 (2): 161–173. doi:10.2217/pme.11.7. ISSN 1741-0541. PMC PMC3118090. PMID 21695041. https://www.futuremedicine.com/doi/10.2217/pme.11.7.
- ↑ Kussmann, Martin; Morine, Melissa J.; Hager, Jörg; Sonderegger, Bernhard; Kaput, Jim (2013). "Perspective: a systems approach to diabetes research". Frontiers in Genetics 4. doi:10.3389/fgene.2013.00205. ISSN 1664-8021. PMC PMC3807566. PMID 24187547. http://journal.frontiersin.org/article/10.3389/fgene.2013.00205/abstract.
- ↑ 25.0 25.1 National Institutes of Health (2020). "2020–2030 Strategic Plan for NIH Nutrition Research: A Report of the NIH Nutrition Research Task Force" (PDF). https://dpcpsi.nih.gov/sites/default/files/2020NutritionStrategicPlan_508.pdf. Retrieved 09 June 2022.
- ↑ Garmany, Armin; Yamada, Satsuki; Terzic, Andre (23 September 2021). "Longevity leap: mind the healthspan gap" (in en). npj Regenerative Medicine 6 (1): 57. doi:10.1038/s41536-021-00169-5. ISSN 2057-3995. PMC PMC8460831. PMID 34556664. https://www.nature.com/articles/s41536-021-00169-5.
- ↑ 27.0 27.1 Afshin, Ashkan; Sur, Patrick John; Fay, Kairsten A.; Cornaby, Leslie; Ferrara, Giannina; Salama, Joseph S; Mullany, Erin C; Abate, Kalkidan Hassen et al. (1 May 2019). "Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017" (in en). The Lancet 393 (10184): 1958–1972. doi:10.1016/S0140-6736(19)30041-8. PMC PMC6899507. PMID 30954305. https://linkinghub.elsevier.com/retrieve/pii/S0140673619300418.
- ↑ National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP). "Health and Economic Costs of Chronic Diseases". Centers for Disease Control and Prevention. https://www.cdc.gov/chronicdisease/about/costs/index.htm. Retrieved 09 June 2022.
- ↑ Wells, Jonathan C; Sawaya, Ana Lydia; Wibaek, Rasmus; Mwangome, Martha; Poullas, Marios S; Yajnik, Chittaranjan S; Demaio, Alessandro (1 January 2020). "The double burden of malnutrition: aetiological pathways and consequences for health" (in en). The Lancet 395 (10217): 75–88. doi:10.1016/S0140-6736(19)32472-9. PMC PMC7613491. PMID 31852605. https://linkinghub.elsevier.com/retrieve/pii/S0140673619324729.
- ↑ Araújo, Joana; Cai, Jianwen; Stevens, June (1 February 2019). "Prevalence of Optimal Metabolic Health in American Adults: National Health and Nutrition Examination Survey 2009–2016" (in en). Metabolic Syndrome and Related Disorders 17 (1): 46–52. doi:10.1089/met.2018.0105. ISSN 1540-4196. https://www.liebertpub.com/doi/10.1089/met.2018.0105.
- ↑ Gkouskou, Kalliopi; Vlastos, Ioannis; Karkalousos, Petros; Chaniotis, Dimitrios; Sanoudou, Despina; Eliopoulos, Aristides G (16 November 2020). "The “Virtual Digital Twins” Concept in Precision Nutrition" (in en). Advances in Nutrition 11 (6): 1405–1413. doi:10.1093/advances/nmaa089. ISSN 2161-8313. PMC PMC7666894. PMID 32770212. https://academic.oup.com/advances/article/11/6/1405/5889940.
- ↑ on behalf of the Swedish Digital Twin Consortium; Björnsson, Bergthor; Borrebaeck, Carl; Elander, Nils; Gasslander, Thomas; Gawel, Danuta R.; Gustafsson, Mika; Jörnsten, Rebecka et al. (1 December 2020). "Digital twins to personalize medicine" (in en). Genome Medicine 12 (1): 4. doi:10.1186/s13073-019-0701-3. ISSN 1756-994X. PMC PMC6938608. PMID 31892363. https://genomemedicine.biomedcentral.com/articles/10.1186/s13073-019-0701-3.
- ↑ Kane, Patrick Bodilly; Bittlinger, Merlin; Kimmelman, Jonathan (1 October 2021). "Individualized therapy trials: navigating patient care, research goals and ethics" (in en). Nature Medicine 27 (10): 1679–1686. doi:10.1038/s41591-021-01519-y. ISSN 1078-8956. https://www.nature.com/articles/s41591-021-01519-y.
- ↑ Swan, Melanie (1 June 2013). "The Quantified Self: Fundamental Disruption in Big Data Science and Biological Discovery" (in en). Big Data 1 (2): 85–99. doi:10.1089/big.2012.0002. ISSN 2167-6461. http://www.liebertpub.com/doi/10.1089/big.2012.0002.
- ↑ 35.0 35.1 Food Forum; Food and Nutrition Board; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine (8 December 2021). Callahan, Emily A.. ed. Challenges and Opportunities for Precision and Personalized Nutrition: Proceedings of a Workshop-in Brief. Washington, D.C.: National Academies Press. doi:10.17226/26407. ISBN 978-0-309-27419-7. https://www.nap.edu/catalog/26407.
- ↑ Crowley, Jennifer; Ball, Lauren; Hiddink, Gerrit Jan (1 September 2019). "Nutrition in medical education: a systematic review" (in en). The Lancet Planetary Health 3 (9): e379–e389. doi:10.1016/S2542-5196(19)30171-8. https://linkinghub.elsevier.com/retrieve/pii/S2542519619301718.
- ↑ Khoury, Muin J.; Iademarco, Michael F.; Riley, William T. (1 March 2016). "Precision Public Health for the Era of Precision Medicine" (in en). American Journal of Preventive Medicine 50 (3): 398–401. doi:10.1016/j.amepre.2015.08.031. PMC PMC4915347. PMID 26547538. https://linkinghub.elsevier.com/retrieve/pii/S074937971500522X.
- ↑ Manyika, J.; Chui, M.; Brown, B. et al. (1 May 2011). "Big data: The next frontier for innovation, competition, and productivity". McKinsey Digital. https://www.mckinsey.com/capabilities/mckinsey-digital/our-insights/big-data-the-next-frontier-for-innovation. Retrieved 09 June 2022.
- ↑ Lee, Yujin; Mozaffarian, Dariush; Sy, Stephen; Huang, Yue; Liu, Junxiu; Wilde, Parke E.; Abrahams-Gessel, Shafika; Jardim, Thiago de Souza Veiga et al. (19 March 2019). Gregg, Ed. ed. "Cost-effectiveness of financial incentives for improving diet and health through Medicare and Medicaid: A microsimulation study" (in en). PLOS Medicine 16 (3): e1002761. doi:10.1371/journal.pmed.1002761. ISSN 1549-1676. PMC PMC6424388. PMID 30889188. https://dx.plos.org/10.1371/journal.pmed.1002761.
- ↑ Wolfenden, Luke; Yoong, Sze Lin (1 September 2021). "Workplace wellness programmes to improve health" (in en). The Lancet Public Health 6 (9): e625. doi:10.1016/S2468-2667(21)00184-5. https://linkinghub.elsevier.com/retrieve/pii/S2468266721001845.
- ↑ Cook, D. J. (1996). "Randomized trials in single subjects: the N of 1 study". Psychopharmacology Bulletin 32 (3): 363–367. ISSN 0048-5764. PMID 8961779. https://pubmed.ncbi.nlm.nih.gov/8961779.
- ↑ Potter, Tilly; Vieira, Rute; de Roos, Baukje (1 June 2021). "Perspective: Application of N-of-1 Methods in Personalized Nutrition Research" (in en). Advances in Nutrition 12 (3): 579–589. doi:10.1093/advances/nmaa173. ISSN 2161-8313. PMC PMC8166550. PMID 33460438. https://academic.oup.com/advances/article/12/3/579/6103955.
- ↑ Moses, Hamilton; Matheson, David H. M.; Cairns-Smith, Sarah; George, Benjamin P.; Palisch, Chase; Dorsey, E. Ray (13 January 2015). "The Anatomy of Medical Research: US and International Comparisons" (in en). JAMA 313 (2): 174. doi:10.1001/jama.2014.15939. ISSN 0098-7484. http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2014.15939.
- ↑ Sharrer, G. Terry (2017), Espina, Virginia, ed., "Personalized Medicine: Ethical Aspects", Molecular Profiling (New York, NY: Springer New York) 1606: 37–50, doi:10.1007/978-1-4939-6990-6_3, ISBN 978-1-4939-6989-0, http://link.springer.com/10.1007/978-1-4939-6990-6_3. Retrieved 2022-12-16
- ↑ Goldberg, Jeanne P.; Sliwa, Sarah A. (1 February 2011). "Communicating actionable nutrition messages: challenges and opportunities" (in en). Proceedings of the Nutrition Society 70 (1): 26–37. doi:10.1017/S0029665110004714. ISSN 0029-6651. https://www.cambridge.org/core/product/identifier/S0029665110004714/type/journal_article.
- ↑ Collier, Roger (14 May 2018). "Containing health myths in the age of viral misinformation" (in en). Canadian Medical Association Journal 190 (19): E578–E578. doi:10.1503/cmaj.180543. ISSN 0820-3946. PMC PMC5953573. PMID 29759962. http://www.cmaj.ca/lookup/doi/10.1503/cmaj.180543.
- ↑ "Precision Nutrition". The Nutrition Source. Harvard T.H. Chan School of Public Health. 2022. https://www.hsph.harvard.edu/nutritionsource/precision-nutrition/.
- ↑ 48.0 48.1 Office of Strategic Coordination - The Common Fund Search the Common Fund Website S. "Nutrition for Precision Health, powered by the All of Us Research Program". National Institutes of Health. https://commonfund.nih.gov/nutritionforprecisionhealth. Retrieved 09 June 2022.
- ↑ National Institutes of Health (20 January 2022). "NIH awards $170 million for precision nutrition study". News Releases. National Institutes of Health. https://www.nih.gov/news-events/news-releases/nih-awards-170-million-precision-nutrition-study. Retrieved 09 June 2022.
- ↑ Ahlgren, Jennie; Nordgren, Anders; Perrudin, Maud; Ronteltap, Amber; Savigny, Jean; van Trijp, Hans; Nordström, Karin; Görman, Ulf (1 July 2013). "Consumers on the Internet: ethical and legal aspects of commercialization of personalized nutrition" (in en). Genes & Nutrition 8 (4): 349–355. doi:10.1007/s12263-013-0331-0. ISSN 1555-8932. PMC PMC3689896. PMID 23471853. http://link.springer.com/10.1007/s12263-013-0331-0.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation and updates to spelling and grammar. In some cases important information was missing from the references, and that information was added.