Difference between revisions of "Journal:The US FDA’s proposed rule on laboratory-developed tests: Impacts on clinical laboratory testing"
Shawndouglas (talk | contribs) (Created stub. Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 37: | Line 37: | ||
==Introduction== | ==Introduction== | ||
On October 3, 2023, the [[Food and Drug Administration|U.S. Food and Drug Administration]] (FDA) released a proposed rule that would regulate [[Laboratory developed test|laboratory-developed tests]] (LDTs) as [[medical device]]s if enacted. [1] The FDA considers ''in vitro'' diagnostics (IVDs) as “tests done on [[sample]]s such as blood or tissue that have been taken from the human body.” [2] These are traditionally manufactured as [[reagent]]s and/or kits that are subject to FDA-clearance/approval and are commercially distributed to [[Clinical laboratory|clinical laboratories]] for [[Medical test|clinical testing]] purposes. LDTs, however, are developed and performed within clinical laboratories and are not commercially distributed outside of the testing site. Additionally, the FDA has defined LDTs as IVDs that are “intended for clinical use and designed, manufactured and used within a single laboratory.” [3] While there is no legislative definition from Congress, nor any current federal regulations that create a legal definition of LDTs in the U.S., the FDA’s definition has been promulgated extensively in non-binding draft guidance documents and public-facing statements. [4, 5] | |||
LDTs are not mentioned in the [[Medical Device Regulation Act|Medical Device Amendments of 1976]] [6]—the law that established the current framework for medical device regulation in the U.S.—nor were LDTs discussed in Congressional hearings prior to the law’s passage. [7, 8] Thus, there is significant legal uncertainty as to whether the FDA has the authority from Congress to advance the current proposed rule. [9] Regardless, FDA leadership has signaled a commitment to move forward with LDT oversight [10], and an April 2024 tentative date for finalization of the proposed rule was posted in the fall 2023 Unified Agenda from the Biden Administration. [11, 12] (The rule was finalized on April 29, 2024.<ref name="FDATakes24">{{cite web |url=https://www.fda.gov/news-events/press-announcements/fda-takes-action-aimed-helping-ensure-safety-and-effectiveness-laboratory-developed-tests |title=FDA Takes Action Aimed at Helping to Ensure the Safety and Effectiveness of Laboratory Developed Tests |publisher=U.S. Food and Drug Administration |date=29 April 2024}}</ref>) | |||
In the U.S., rulemaking from federal agencies must follow requirements outlined in the Administrative Procedure Act. [13] The FDA’s proposed rule on LDTs is considered "notice-and-comment" rulemaking, where the public is notified of a proposed rule and provided with the opportunity to provide open comments, and the agency is required to consider this feedback from the public in its deliberation and rulemaking. [14] The public comment period for the FDA’s proposed rule was limited to 60 days, and it officially closed on December 4, 2023, with approximately 6,700 comments submitted. [15] The FDA declined numerous requests for extensions of the public comment period. [16] These requests included a letter signed by leaders of 89 laboratories and professional organizations [17], as well as a request from the American Medical Association House of Delegates. [18] Extension of public comment periods can provide members of the public more time to evaluate the implication of a proposed rule, and they are often granted by agencies for rules with significant potential impact to the public. The FDA has hosted no public workshops or hearings on LDT oversight since 2015. [19] | |||
An important aspect of the public comment process is to assist a federal agency in understanding whether different sectors of the public support or oppose a proposed rule and for which reasons. While the Federal Register’s comment submission system for the proposed rule on LDTs included a drop-down menu for submitters to select the industry, corporate type, career, or setting most applicable to them—presumably to assist federal agencies in categorizing comments by sector—"clinical laboratory" or "clinical laboratorian" were not available options, even though this setting and career type is most impacted by the proposed rule. As such, it will be difficult for the FDA to conduct an accurate quantitative analysis of public opinion in a key sector directly impacted by its proposed rule. | |||
To provide a quantitative assessment of clinical laboratorian opinions on the FDA’s proposed rule and its anticipated impact on clinical laboratory operations and patient care, a survey was distributed in February 2024 to customer contacts of ARUP Laboratories. ARUP is a national, nonprofit clinical laboratory enterprise of the University of Utah Department of Pathology, with community [[hospital]] and academic medical center laboratory customers across all 50 states. [20] As such, recipients of this survey are directly involved in clinical laboratory testing and have occupational roles for which FDA-cleared/approved IVDs and LDTs are relevant and familiar. Survey results demonstrate that there is strong opposition among clinical laboratory respondents to the proposed rule and significant concerns regarding its anticipated negative impacts on clinical laboratory testing and patient care across laboratories. | |||
==Materials and methods== | |||
A ten-item questionnaire regarding the potential impact of the FDA’s proposed rule on LDTs was developed (see Survey Questionnaire). The study received an exemption determination (Category 2) from the University of Utah Institutional Review Board (IRB 00174067). [21] An invitation to participate in this survey was distributed to customer contact email addresses from ARUP’s [[customer relationship management]] system (CRM) (Salesforce; San Francisco, CA). The survey was opened on January 31, 2024 and closed on February 21, 2024. Documentation of informed consent was waived by the IRB for this exempt protocol. A cover letter outlining the study was provided in the invitation email for this anonymous questionnaire, and individuals who chose to not complete the survey could disregard this invitation. Individual survey links could only be used for one submission. As ARUP Laboratories operates the University of Utah Health clinical laboratories and also serves as the health system’s primary reference laboratory, surveys were not distributed to either ARUP or University of Utah Health email addresses to avoid the potential for respondent bias regarding expectations of reference laboratory services. Contacts at national [[Reference laboratory|reference laboratories]] were also excluded to avoid similar bias regarding reference laboratory services. [[Medical research|Research trial]] contacts were also excluded as not all studies require [[Clinical Laboratory Improvement Amendments]] (CLIA) certification. The survey was administered using the Experience Management XM platform (Qualtrics; Provo, UT), and no identifiers were collected from survey respondents. Respondent institution type (i.e., community hospital, academic hospital, pediatric hospital, Veterans Administration / federal hospital, independent reference laboratory, pathology group or clinic) and institution location (i.e., U.S. state, territory, or country) was available for a subset of customers in the CRM for summary analysis. A geographic map of institutions in each U.S. state was created using a template from SlideQuest<ref name="TSQHome">{{cite web |url=https://theslidequest.com/ |title=SlideQuest |publisher=TheSlideQuest |date=2024}}</ref> in PowerPoint (Microsoft 365; Redmond, WA). | |||
Data was tabulated within Qualtrics and further analyzed and displayed using Excel (Microsoft 365; Redmond, WA). Percentages are rounded to whole numbers throughout the manuscript. Statistical analyses were performed using [[R (programming language)|R]] Statistical Software (v4.3.3; R Core Team, 2024). Thematic analysis of free text comments was also conducted and included both semantic themes (e.g., the precise wording of a comment) and latent themes (e.g., an underlying concept in a comment) [22]. Respondent text was assigned to thematic categories and tabulated in Excel, as previously described. [23] Respondent comments displayed in the manuscript were edited for spelling and minor grammatical corrections only. | |||
==Results== | |||
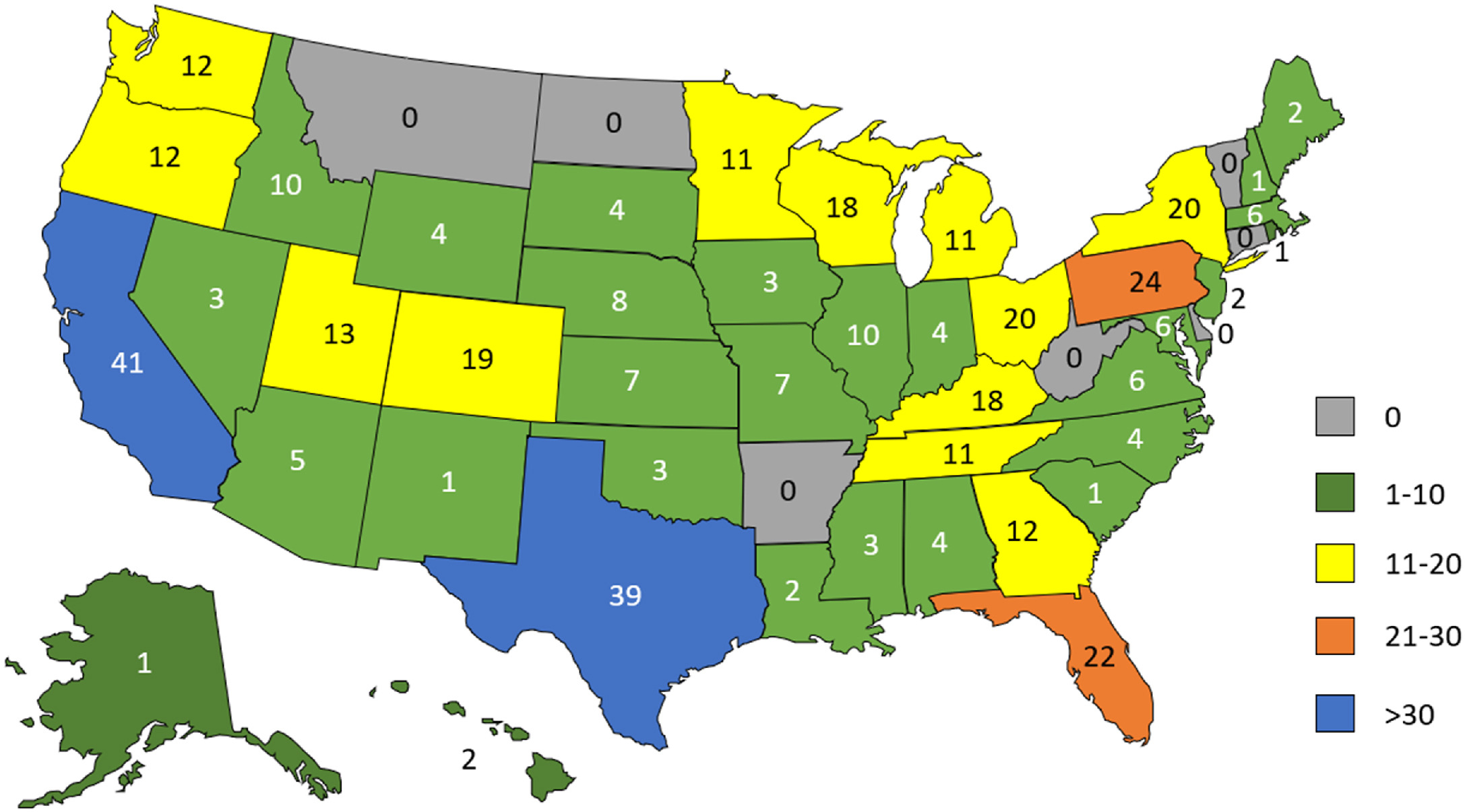
Of survey invitations distributed by email to 15,513 individuals, 532 questionnaires (3% response rate) were either fully or partially completed. Information on respondent institution’s state, territory, or country was known for 429 respondents (81%), with 421 institutions in this subset (98%) located in the U.S. across 45 states. Eight additional respondents were affiliated with institutions outside the U.S. Distribution of respondent institution location by U.S. state is shown in Figure 1. | |||
[[File:Fig1 Smith PracLabMed2024 InPress.jpg|700px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="700px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Figure 1.''' Respondent institution by U.S. state. Map showing respondent institution by state (where available).</blockquote> | |||
|- | |||
|} | |||
|} | |||
Information on respondent institution type was available for 327 respondents (62%). Distribution of these institutions by category is shown in Table 1. | |||
{| | |||
| style="vertical-align:top;" | | |||
{| class="wikitable" border="1" cellpadding="5" cellspacing="0" width="100%" | |||
|- | |||
| colspan="3" style="background-color:white; padding-left:10px; padding-right:10px;" |'''Table 1.''' Respondent institution by category. | |||
|- | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Institution category | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Number | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Percent | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Hospital (community) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |179 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |55% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Hospital (academic) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |64 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |20% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Independent reference laboratory | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |40 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |12% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Hospital (children’s) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |25 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |8% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Hospital (Veterans Administration/federal) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |10 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |3% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Pathology group or clinic | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |9 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |3% | |||
|- | |||
|} | |||
|} | |||
Respondents were asked in the questionnaire to specify a job that best describes their roles within their respective organization. A categorized list of respondent job roles is shown in Table 2. The most common job categories selected by respondents included lab manager or supervisor (153, 29%), lab director (97, 18%), lab employee (med tech, lab tech, lab assistant; 83, 16%), and medical director, pathologist, physician, clinician, or PhD scientist (68, 13%). | |||
{| | |||
| style="vertical-align:top;" | | |||
{| class="wikitable" border="1" cellpadding="5" cellspacing="0" width="100%" | |||
|- | |||
| colspan="3" style="background-color:white; padding-left:10px; padding-right:10px;" |'''Table 2.''' Job categories of survey respondents. CEO, chief executive officer; CFO, chief financial officer; IT, information technology; LIS, [[laboratory information system]]. | |||
|- | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Job category | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Number | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Percent | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Lab manager or supervisor | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |153 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |29% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Lab director | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |97 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |18% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Lab employee (med tech / lab tech / lab assistant) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |83 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |16% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Medical director, pathologist, physician, clinician, or PhD scientist | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |68 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |13% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Send-out / referral testing | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |29 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |6% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Quality and compliance | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |25 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |5% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Executive (CEO, CFO, etc.) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |23 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |4% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |IT / LIS | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |13 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |2% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Customer service / support service | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |10 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |2% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Office: executive assistant, administrative assistant, etc. | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |4 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |1% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Specimen processing / receiving | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Supply chain / ancillary services | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Other | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |25 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |5% | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |'''Total''' | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |'''532''' | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
|} | |||
|} | |||
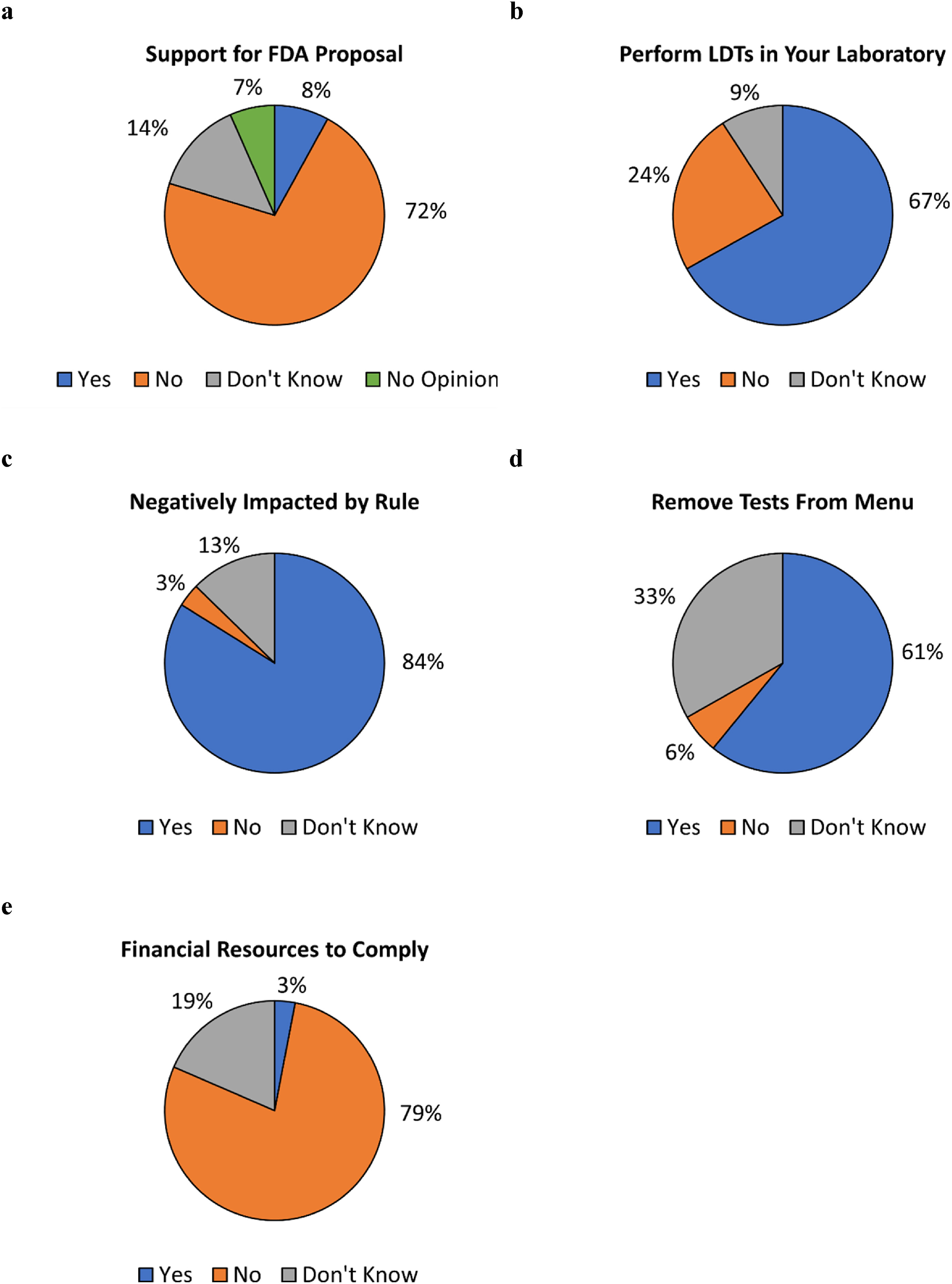
503 respondents then answered a question regarding whether they support the FDA’s proposed rule to regulate LDTs as medical devices. A majority (360, 72%) responded "no," 41 (8%) responded "yes" and 102 (20%) responded that they either had no opinion (69, 14%) or did not know whether they support the proposed rule (33, 7%) (Fig 2a). Distribution of responses according to job category is presented in Table 3. The highest percentage of "no" responses were in the "medical director, pathologist, physician, clinician, or PhD scientist" job category (91%), the "quality and compliance" job category (91%), the "executive (CEO, CFO, etc.)" job category (86%), and the "lab director" job category (83%). The Fisher’s exact test was used to examine the differences in response by job category. Although the test indicated statistical differences within the dataset, pairwise examination of responses by job category found no statistical differences. These conflicting results may be due to a loss of power due to smaller sample sizes in the pairwise analysis. | |||
[[File:Fig2 Smith PracLabMed2024 InPress.jpg|600px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="700px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Figure 2.''' Responses to survey questions. Respondents were asked ('''a''') whether they support the FDA proposal to regulate LDTs, ('''b''') whether they perform LDTs within their laboratories, ('''c''') if their laboratories would be negatively impacted by the proposed rule if enacted, ('''d''') if they anticipate having to remove tests from their laboratory menus if the proposed rule is enacted, and ('''e''') whether they have the financial resources to pay for FDA user fees. Questions for ('''c'''), ('''d'''), and ('''e''') were asked only of respondents whose laboratories performed LDTs.</blockquote> | |||
|- | |||
|} | |||
|} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| class="wikitable" border="1" cellpadding="5" cellspacing="0" width="70%" | |||
|- | |||
| colspan="10" style="background-color:white; padding-left:10px; padding-right:10px;" |'''Table 3.''' Responses to "Do Your Support the FDA's Proposed Rule to Regulate Laboratory-Developed Tests and Medical Devices?" CEO, chief executive officer; CFO, chief financial officer; IT, information technology; LIS, [[laboratory information system]]. | |||
|- | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" rowspan="2"|Job category | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" colspan="2"|Yes | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" colspan="2"|No | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" colspan="2"|Don't know | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" colspan="2"|No opinion | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Total | |||
|- | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Number | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |% | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Number | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |% | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Number | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |% | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Number | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |% | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Number | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Lab manager or supervisor | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |16 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |11 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |101 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |68 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |8 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |5 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |24 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |16 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |149 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Lab director | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |4 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |4 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |78 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |83 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |4 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |4 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |8 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |9 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |94 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Lab employee (med tech / lab tech / lab assistant) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |10 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |13 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |45 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |58 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |10 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |13 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |13 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |17 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |78 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Medical director, pathologist, physician, clinician, or PhD scientist | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |62 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |91 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |2 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |3 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |4 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |6 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |68 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Send-out / referral testing | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |3 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |12 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |11 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |44 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |4 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |16 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |7 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |28 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |25 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Quality and compliance | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |5 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |20 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |91 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |5 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |22 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Executive (CEO, CFO, etc.) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |19 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |86 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |3 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |14 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |22 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |IT / LIS | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |2 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |18 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |3 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |27 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |2 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |18 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |4 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |36 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |11 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Customer service / support service | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |2 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |22 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |6 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |67 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |11 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |9 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Office: executive assistant, administrative assistant, etc. | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |2 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |67 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |33 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |3 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Specimen processing / receiving | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |100 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |1 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Supply chain / ancillary services | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |100 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |1 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Other | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |14 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |70 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |3 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |15 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |3 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |15 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |20 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |'''Total''' | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |'''41''' | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |'''360''' | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |'''33''' | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |'''69''' | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |'''503''' | |||
|- | |||
|} | |||
|} | |||
489 respondents then answered a question regarding whether their laboratory performs LDTs. 327 (67%) responded "yes," 117 (24%) responded "no," and 45 (9%) responded "don’t know" (Fig 2b). As the survey was intended to assess how laboratories that perform LDTs would respond to the proposed FDA rule if enacted, a response of "no" or "don’t know" ended the survey for those respective respondents. | |||
Respondents whose laboratories perform LDTs were then asked whether the FDA’s proposed rule would negatively impact their laboratories (''n''=322 responses). 270 (84%) responded "yes," 11 (3%) responded "no," and 41 (13%) responded "don’t know" (Fig 2c). Respondents were also asked whether they anticipate having to remove tests from their menu if the proposed rule is enacted (''n''=304 responses). A majority, 185 (61%) responded 'yes,' 101 (33%) responded 'don’t know,' and 18 (6%) responded 'no' (Fig 2d). Respondents were also asked whether their laboratories have the financial resources to pay for FDA user fees, with fiscal year 2024 medical device user fees displayed in the question stem: $21,760 per “moderate risk” 510(k) submission and $483,560 per “high risk” premarket authorization submission [24] (''n''=303 responses). 238 (79%) responded "no," 56 (19%) responded "don’t know," and 9 (3%) responded "yes" (Fig 2e). | |||
High levels of concern regarding the potential impact of the proposed rule were expressed by respondents in this survey (Fig 3), with answers of "extremely concerned" or "very concerned" provided across all topics queried, including patient access to essential testing (269 of 305, 88%), availability of financial resources to comply with the proposed regulations (264 of 305, 87%), impact on innovation (261 of 306, 86%), future laboratory send-out costs (252 of 304, 83%), increase in test prices (248 of 303, 82%), availability of personnel resources to comply with the proposed regulations (247 of 305, 81%), and FDA’s ability to implement the proposed regulations (245 of 305, 80%). Only a small percentage of respondents (≤6%) chose either "not concerned at all" or "slightly concerned" for any of the topics queried. | |||
==References== | ==References== | ||
| Line 43: | Line 381: | ||
==Notes== | ==Notes== | ||
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. No other changes have been made, in accord with the "NoDerivs" portion of the license. | This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. A note (and citation) indicating that the final rule was announced at the end of April was added. No other changes have been made, in accord with the "NoDerivs" portion of the license. | ||
<!--Place all category tags here--> | <!--Place all category tags here--> | ||
Revision as of 20:05, 26 May 2024
| Full article title | The US FDA’s proposed rule on laboratory-developed tests: Impacts on clinical laboratory testing |
|---|---|
| Journal | Practical Laboratory Medicine |
| Author(s) | Smith, Leslie; Carricaburu, Lisa A.; Genzen, Jonathan R. |
| Author affiliation(s) | ARUP Laboratories, University of Utah Health |
| Primary contact | Email: jonathan dot genzen at path dot utah dot edu |
| Year published | 2024 |
| Volume and issue | In press |
| Article # | e00407 |
| DOI | 10.1016/j.plabm.2024.e00407 |
| ISSN | 2352-5517 |
| Distribution license | Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International |
| Website | https://www.sciencedirect.com/science/article/pii/S2352551724000532 |
| Download | https://www.sciencedirect.com/science/article/pii/S2352551724000532/pdfft (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Objectives: To solicit quantifiable feedback from clinical laboratorians on the U.S. Food and Drug Administration (FDA) proposed rule to regulate laboratory-developed tests (LDTs) as medical devices.
Design and methods: A ten-item questionnaire was developed and submitted to clinical laboratory customers of ARUP Laboratories, a national nonprofit clinical laboratory of the University of Utah Department of Pathology.
Results: Of 503 clinical laboratory respondents, only 41 (8%) support the FDA’s proposed rule. 67% of respondents work in laboratories that perform LDTs and were therefore asked additional questions regarding the proposed rule. 84% of these respondents believe that the proposed rule will negatively impact their laboratories, while only 3% believe that they have the financial resources to pay for FDA user fees. 61% of respondents anticipate removing tests from their laboratory menus if the proposed rule is enacted, while an additional 33% indicated that they do not yet know. Only 11% of respondents believe that they would pursue FDA submissions for all of their existing LDTs if the final rule is enacted. The vast majority of respondents (>80%) were either "extremely concerned" or "very concerned" about the impact of the proposed rule on patient access to essential testing, financial and personnel resources to comply, innovation, the FDA’s ability to implement the rule, and send-out costs and test prices.
Conclusions: The majority of clinical laboratorians surveyed do not support the FDA’s proposed rule on LDTs and report having insufficient resources to comply with the rule if it is enacted.
Keywords: laboratory-developed tests, Food and Drug Administration, Clinical Laboratory Improvement Amendments, clinical laboratory regulations
Introduction
On October 3, 2023, the U.S. Food and Drug Administration (FDA) released a proposed rule that would regulate laboratory-developed tests (LDTs) as medical devices if enacted. [1] The FDA considers in vitro diagnostics (IVDs) as “tests done on samples such as blood or tissue that have been taken from the human body.” [2] These are traditionally manufactured as reagents and/or kits that are subject to FDA-clearance/approval and are commercially distributed to clinical laboratories for clinical testing purposes. LDTs, however, are developed and performed within clinical laboratories and are not commercially distributed outside of the testing site. Additionally, the FDA has defined LDTs as IVDs that are “intended for clinical use and designed, manufactured and used within a single laboratory.” [3] While there is no legislative definition from Congress, nor any current federal regulations that create a legal definition of LDTs in the U.S., the FDA’s definition has been promulgated extensively in non-binding draft guidance documents and public-facing statements. [4, 5]
LDTs are not mentioned in the Medical Device Amendments of 1976 [6]—the law that established the current framework for medical device regulation in the U.S.—nor were LDTs discussed in Congressional hearings prior to the law’s passage. [7, 8] Thus, there is significant legal uncertainty as to whether the FDA has the authority from Congress to advance the current proposed rule. [9] Regardless, FDA leadership has signaled a commitment to move forward with LDT oversight [10], and an April 2024 tentative date for finalization of the proposed rule was posted in the fall 2023 Unified Agenda from the Biden Administration. [11, 12] (The rule was finalized on April 29, 2024.[1])
In the U.S., rulemaking from federal agencies must follow requirements outlined in the Administrative Procedure Act. [13] The FDA’s proposed rule on LDTs is considered "notice-and-comment" rulemaking, where the public is notified of a proposed rule and provided with the opportunity to provide open comments, and the agency is required to consider this feedback from the public in its deliberation and rulemaking. [14] The public comment period for the FDA’s proposed rule was limited to 60 days, and it officially closed on December 4, 2023, with approximately 6,700 comments submitted. [15] The FDA declined numerous requests for extensions of the public comment period. [16] These requests included a letter signed by leaders of 89 laboratories and professional organizations [17], as well as a request from the American Medical Association House of Delegates. [18] Extension of public comment periods can provide members of the public more time to evaluate the implication of a proposed rule, and they are often granted by agencies for rules with significant potential impact to the public. The FDA has hosted no public workshops or hearings on LDT oversight since 2015. [19]
An important aspect of the public comment process is to assist a federal agency in understanding whether different sectors of the public support or oppose a proposed rule and for which reasons. While the Federal Register’s comment submission system for the proposed rule on LDTs included a drop-down menu for submitters to select the industry, corporate type, career, or setting most applicable to them—presumably to assist federal agencies in categorizing comments by sector—"clinical laboratory" or "clinical laboratorian" were not available options, even though this setting and career type is most impacted by the proposed rule. As such, it will be difficult for the FDA to conduct an accurate quantitative analysis of public opinion in a key sector directly impacted by its proposed rule.
To provide a quantitative assessment of clinical laboratorian opinions on the FDA’s proposed rule and its anticipated impact on clinical laboratory operations and patient care, a survey was distributed in February 2024 to customer contacts of ARUP Laboratories. ARUP is a national, nonprofit clinical laboratory enterprise of the University of Utah Department of Pathology, with community hospital and academic medical center laboratory customers across all 50 states. [20] As such, recipients of this survey are directly involved in clinical laboratory testing and have occupational roles for which FDA-cleared/approved IVDs and LDTs are relevant and familiar. Survey results demonstrate that there is strong opposition among clinical laboratory respondents to the proposed rule and significant concerns regarding its anticipated negative impacts on clinical laboratory testing and patient care across laboratories.
Materials and methods
A ten-item questionnaire regarding the potential impact of the FDA’s proposed rule on LDTs was developed (see Survey Questionnaire). The study received an exemption determination (Category 2) from the University of Utah Institutional Review Board (IRB 00174067). [21] An invitation to participate in this survey was distributed to customer contact email addresses from ARUP’s customer relationship management system (CRM) (Salesforce; San Francisco, CA). The survey was opened on January 31, 2024 and closed on February 21, 2024. Documentation of informed consent was waived by the IRB for this exempt protocol. A cover letter outlining the study was provided in the invitation email for this anonymous questionnaire, and individuals who chose to not complete the survey could disregard this invitation. Individual survey links could only be used for one submission. As ARUP Laboratories operates the University of Utah Health clinical laboratories and also serves as the health system’s primary reference laboratory, surveys were not distributed to either ARUP or University of Utah Health email addresses to avoid the potential for respondent bias regarding expectations of reference laboratory services. Contacts at national reference laboratories were also excluded to avoid similar bias regarding reference laboratory services. Research trial contacts were also excluded as not all studies require Clinical Laboratory Improvement Amendments (CLIA) certification. The survey was administered using the Experience Management XM platform (Qualtrics; Provo, UT), and no identifiers were collected from survey respondents. Respondent institution type (i.e., community hospital, academic hospital, pediatric hospital, Veterans Administration / federal hospital, independent reference laboratory, pathology group or clinic) and institution location (i.e., U.S. state, territory, or country) was available for a subset of customers in the CRM for summary analysis. A geographic map of institutions in each U.S. state was created using a template from SlideQuest[2] in PowerPoint (Microsoft 365; Redmond, WA).
Data was tabulated within Qualtrics and further analyzed and displayed using Excel (Microsoft 365; Redmond, WA). Percentages are rounded to whole numbers throughout the manuscript. Statistical analyses were performed using R Statistical Software (v4.3.3; R Core Team, 2024). Thematic analysis of free text comments was also conducted and included both semantic themes (e.g., the precise wording of a comment) and latent themes (e.g., an underlying concept in a comment) [22]. Respondent text was assigned to thematic categories and tabulated in Excel, as previously described. [23] Respondent comments displayed in the manuscript were edited for spelling and minor grammatical corrections only.
Results
Of survey invitations distributed by email to 15,513 individuals, 532 questionnaires (3% response rate) were either fully or partially completed. Information on respondent institution’s state, territory, or country was known for 429 respondents (81%), with 421 institutions in this subset (98%) located in the U.S. across 45 states. Eight additional respondents were affiliated with institutions outside the U.S. Distribution of respondent institution location by U.S. state is shown in Figure 1.
|
Information on respondent institution type was available for 327 respondents (62%). Distribution of these institutions by category is shown in Table 1.
| ||||||||||||||||||||||||
Respondents were asked in the questionnaire to specify a job that best describes their roles within their respective organization. A categorized list of respondent job roles is shown in Table 2. The most common job categories selected by respondents included lab manager or supervisor (153, 29%), lab director (97, 18%), lab employee (med tech, lab tech, lab assistant; 83, 16%), and medical director, pathologist, physician, clinician, or PhD scientist (68, 13%).
| ||||||||||||||||||||||||||||||||||||||||||||||||
503 respondents then answered a question regarding whether they support the FDA’s proposed rule to regulate LDTs as medical devices. A majority (360, 72%) responded "no," 41 (8%) responded "yes" and 102 (20%) responded that they either had no opinion (69, 14%) or did not know whether they support the proposed rule (33, 7%) (Fig 2a). Distribution of responses according to job category is presented in Table 3. The highest percentage of "no" responses were in the "medical director, pathologist, physician, clinician, or PhD scientist" job category (91%), the "quality and compliance" job category (91%), the "executive (CEO, CFO, etc.)" job category (86%), and the "lab director" job category (83%). The Fisher’s exact test was used to examine the differences in response by job category. Although the test indicated statistical differences within the dataset, pairwise examination of responses by job category found no statistical differences. These conflicting results may be due to a loss of power due to smaller sample sizes in the pairwise analysis.
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
489 respondents then answered a question regarding whether their laboratory performs LDTs. 327 (67%) responded "yes," 117 (24%) responded "no," and 45 (9%) responded "don’t know" (Fig 2b). As the survey was intended to assess how laboratories that perform LDTs would respond to the proposed FDA rule if enacted, a response of "no" or "don’t know" ended the survey for those respective respondents.
Respondents whose laboratories perform LDTs were then asked whether the FDA’s proposed rule would negatively impact their laboratories (n=322 responses). 270 (84%) responded "yes," 11 (3%) responded "no," and 41 (13%) responded "don’t know" (Fig 2c). Respondents were also asked whether they anticipate having to remove tests from their menu if the proposed rule is enacted (n=304 responses). A majority, 185 (61%) responded 'yes,' 101 (33%) responded 'don’t know,' and 18 (6%) responded 'no' (Fig 2d). Respondents were also asked whether their laboratories have the financial resources to pay for FDA user fees, with fiscal year 2024 medical device user fees displayed in the question stem: $21,760 per “moderate risk” 510(k) submission and $483,560 per “high risk” premarket authorization submission [24] (n=303 responses). 238 (79%) responded "no," 56 (19%) responded "don’t know," and 9 (3%) responded "yes" (Fig 2e).
High levels of concern regarding the potential impact of the proposed rule were expressed by respondents in this survey (Fig 3), with answers of "extremely concerned" or "very concerned" provided across all topics queried, including patient access to essential testing (269 of 305, 88%), availability of financial resources to comply with the proposed regulations (264 of 305, 87%), impact on innovation (261 of 306, 86%), future laboratory send-out costs (252 of 304, 83%), increase in test prices (248 of 303, 82%), availability of personnel resources to comply with the proposed regulations (247 of 305, 81%), and FDA’s ability to implement the proposed regulations (245 of 305, 80%). Only a small percentage of respondents (≤6%) chose either "not concerned at all" or "slightly concerned" for any of the topics queried.
References
- ↑ "FDA Takes Action Aimed at Helping to Ensure the Safety and Effectiveness of Laboratory Developed Tests". U.S. Food and Drug Administration. 29 April 2024. https://www.fda.gov/news-events/press-announcements/fda-takes-action-aimed-helping-ensure-safety-and-effectiveness-laboratory-developed-tests.
- ↑ "SlideQuest". TheSlideQuest. 2024. https://theslidequest.com/.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. A note (and citation) indicating that the final rule was announced at the end of April was added. No other changes have been made, in accord with the "NoDerivs" portion of the license.