Difference between revisions of "Template:Article of the week"
From LIMSWiki
Jump to navigationJump to searchShawndouglas (talk | contribs) (Updated article of the week text) |
Shawndouglas (talk | contribs) (Updated article of the week text.) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File: | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig3 Johnson JofCannRes23 5.png|240px]]</div> | ||
'''"[[Journal:The | '''"[[Journal:Potency and safety analysis of hemp-derived delta-9 products: The hemp vs. cannabis demarcation problem|Potency and safety analysis of hemp-derived delta-9 products: The hemp vs. cannabis demarcation problem]]"''' | ||
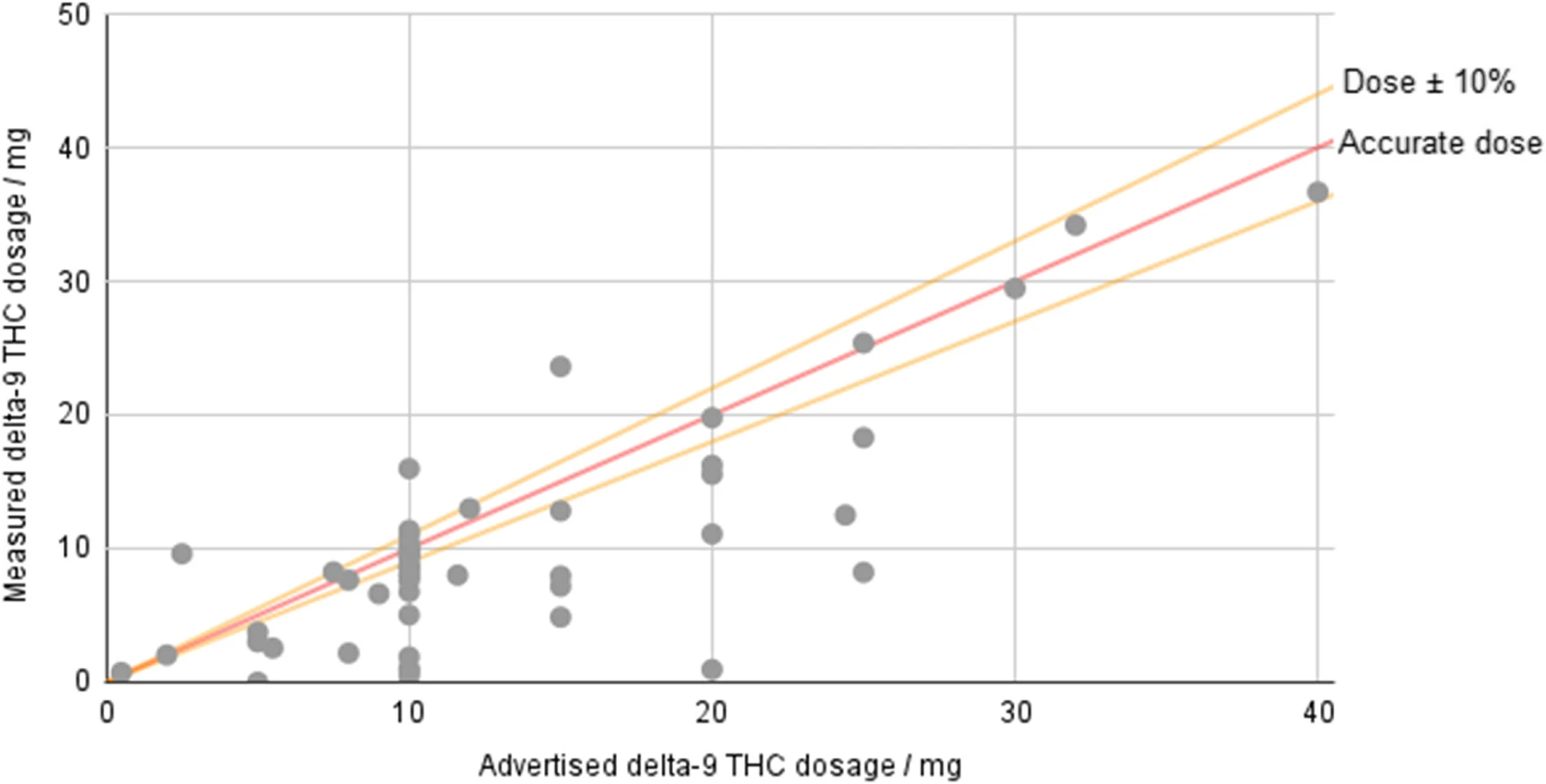
[[Hemp]]-derived [[Tetrahydrocannabinol|delta-9-tetrahydrocannabinol]] (Δ<sup>9</sup>-THC) products are freely available for sale across much of the USA, but the federal legislation allowing their sale places only minimal requirements on companies. Products must contain no more than 0.3% Δ<sup>9</sup>-THC by dry weight, but no limit is placed on overall dosage, and there is no requirement that products derived from hemp-based Δ<sup>9</sup>-THC be tested. However, some states—such as Colorado—specifically prohibit products created by “chemically modifying” a natural hemp component. Fifty-three hemp-derived Δ<sup>9</sup>-THC products were ordered and submitted to InfiniteCAL [[laboratory]] for analysis ... ('''[[Journal:Potency and safety analysis of hemp-derived delta-9 products: The hemp vs. cannabis demarcation problem|Full article...]]''')<br /> | |||
''Recently featured'': | ''Recently featured'': | ||
{{flowlist | | {{flowlist | | ||
* [[Journal:The NOMAD Artificial Intelligence Toolkit: Turning materials science data into knowledge and understanding|The NOMAD Artificial Intelligence Toolkit: Turning materials science data into knowledge and understanding]] | |||
* [[Journal:Quality control in the clinical biochemistry laboratory: A glance|Quality control in the clinical biochemistry laboratory: A glance]] | * [[Journal:Quality control in the clinical biochemistry laboratory: A glance|Quality control in the clinical biochemistry laboratory: A glance]] | ||
* [[Journal:Shared metadata for data-centric materials science|Shared metadata for data-centric materials science]] | * [[Journal:Shared metadata for data-centric materials science|Shared metadata for data-centric materials science]] | ||
}} | }} | ||
Revision as of 19:02, 13 February 2024
Hemp-derived delta-9-tetrahydrocannabinol (Δ9-THC) products are freely available for sale across much of the USA, but the federal legislation allowing their sale places only minimal requirements on companies. Products must contain no more than 0.3% Δ9-THC by dry weight, but no limit is placed on overall dosage, and there is no requirement that products derived from hemp-based Δ9-THC be tested. However, some states—such as Colorado—specifically prohibit products created by “chemically modifying” a natural hemp component. Fifty-three hemp-derived Δ9-THC products were ordered and submitted to InfiniteCAL laboratory for analysis ... (Full article...)
Recently featured: