Journal:Potency and safety analysis of hemp-derived delta-9 products: The hemp vs. cannabis demarcation problem
| Full article title | Potency and safety analysis of hemp-derived delta-9 products: The hemp vs. cannabis demarcation problem |
|---|---|
| Journal | Journal of Cannabis Research |
| Author(s) | Johnson, Lee; Malone, Marc; Paulson, Erik; Swider, Josh; Marelius, David; Andersen, Susan; Black, Dominic |
| Author affiliation(s) | CBD Oracle, Infinite Chemical Analysis Labs |
| Primary contact | Email: lee at cbdoracle dot com |
| Year published | 2023 |
| Volume and issue | 5 |
| Article # | 29 |
| DOI | 10.1186/s42238-023-00197-6 |
| ISSN | 2522-5782 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://jcannabisresearch.biomedcentral.com/articles/10.1186/s42238-023-00197-6 |
| Download | https://jcannabisresearch.biomedcentral.com/counter/pdf/10.1186/s42238-023-00197-6.pdf (PDF) |
Abstract
Background: Hemp-derived delta-9-tetrahydrocannabinol (Δ9-THC) products are freely available for sale across much of the USA, but the federal legislation allowing their sale places only minimal requirements on companies. Products must contain no more than 0.3% Δ9-THC by dry weight, but no limit is placed on overall dosage, and there is no requirement that products derived from hemp-based Δ9-THC be tested. However, some states—such as Colorado—specifically prohibit products created by “chemically modifying” a natural hemp component.
Methods: Fifty-three hemp-derived Δ9-THC products were ordered and submitted to InfiniteCAL laboratory for analysis. The lab analysis considered potency, the presence of impurities, and whether the Δ9-THC present was natural or converted from cannabidiol (CBD). The presence of age verification, company-conducted testing, and warning labels was also considered.
Results: While 96.2% of products were under the legal Δ9-THC limit, 66.0% differed from their stated dosage by more than 10%, and although 84.9% provided a lab report to customers, 71.1% of these did not check for impurities. Additionally, 49% of products converted CBD to THC to achieve their levels, and only 15.1% performed age verification at checkout.
Conclusions: Despite some positive findings, the results show that hemp-derived Δ9-THC companies offer inaccurately labeled products that contain more THC than would be allowed in adult-use states. This raises serious issues around consumer safety, and consent when consuming intoxicating products. Steps to boost accountability for companies must be considered by either the industry or lawmakers if intoxicating hemp products are to safely remain on the market.
Keywords: hemp, Δ9-THC, Farm Bill, Agriculture Improvement Act, cannabinoid potency
Background
Delta-9-tetrahydrocannabinol (Δ9-THC) is the primary psychoactive component of the Cannabis sativa L. plant[1], with other cannabinoids like cannabidiol (CBD) attracting attention for their therapeutic properties[2] in recent years.[3] While both cannabinoids have medical applications, Δ9-THC has largely been associated with recreational use. Until 2012[4], the prohibition of the recreational use of cannabis in the United States has made it essentially impossible to obtain legally, except through certain medical channels.
However, things changed when the Agriculture Improvement Act of 2018 (a.k.a. the “Farm Bill”) made industrial hemp legal at the federal level.[5] The legislation allowed for an explosion of CBD products, but there were unintended consequences. The Farm Bill removed the cannabinoids in hemp from the definition of "marijuana" in the Controlled Substances Act and defined hemp as containing less than 0.3% Δ9-THC by dry weight.[6] This allowed non-intoxicating CBD oils, for example, to be sold freely. However, loopholes quickly emerged, such as ignoring the matter of Δ8-THC, another psychoactive compound much like Δ9-THC except with less potent and long-lasting effects[7] and less binding affinity for the CB1 receptor.[8] Since it is a natural component of hemp, provided that products containing it have less than 0.3% Δ9-THC by dry weight, they can contain as much Δ8-THC as they want. Some states have taken action to stop the sale and distribution of Δ8-THC[6], but new loopholes (for example, the increase in products with hexahydrocannabinol [HHC][9]) are identified more quickly than lawmakers can close them.
While Δ8-THC is present in negligible amounts in the Cannabis plant, virtually all products sold to consumers use Δ8-THC produced from CBD[8] by cyclization (the closure of a ring after an acid-catalyzed activation of a double bond).[10] This creates potential legal issues at the federal level (because it may render it “synthetic” THC), but the conversion process itself has also been a target of state-level legislation.[11][12]
Hemp-derived Δ9-THC products were devised through a very simple application of the Farm Bill’s 0.3% by dry weight limit. A 10 g gummy can contain roughly 10 g × 0.3% = 0.03 g = 30 mg of Δ9-THC and still be within the legal limit. In contrast, intoxicating cannabis edibles in legal states like California and Colorado tend to contain just 5 mg or 10 mg Δ9-THC per serving.[13][14] As an unavoidable consequence of the law as it is currently written, intoxicating “hemp” Δ9-THC products are widely available in most states.
There are many potential issues with this; however, the biggest is the minimal regulations imposed on these “hemp” companies, especially in comparison to the regulations of legal cannabis markets. For instance, in California[15], each product must be lab tested for cannabinoid potency, residual pesticides, foreign material, heavy metals, microbial impurities, mycotoxins, moisture content, and residual solvents, and packaging must be child-resistant, tamper-evident, and resealable, containing a cannabis universal symbol and numerous other pieces of information, such as a batch number and a full ingredient listing. These and similar regulations protect consumers in states with legal cannabis, but are not a requirement for hemp under the Farm Bill.
Since hemp-derived Δ9-THC products are intoxicating, many people argue that they should meet similar standards to edibles in states like California and Colorado[16], and be subject to the same requirements for things like warning labels and child-safe packaging. As with Δ8-THC products, it is also possible that some of the Δ9-THC in hemp products is created through cyclization, and consequently may be impacted by existing state legislation.
This study aims to investigate the hemp-derived Δ9-THC market with this in mind. In particular, we aim to determine whether companies remain within legal limits, whether the stated dosages are accurate, whether the Δ9-THC was produced by cyclization, and whether companies performed safety testing on products and made sufficient effort to prevent minors from purchasing them.
Methods
Sample size and product selection
For the lab study and market analysis, we first identified and purchased a selection of the most popular products online from different brands. To identify brands, Google searches for “hemp delta-9 thc edibles,” “hemp delta-9 thc tinctures,” “hemp delta-9 thc vapes,” “hemp delta-9 thc products,” “full spectrum CBD + THC product,” and “compliant delta-9 thc product” were performed, and the first 20 pages of results (200 search results total) were reviewed. The relevant commercial results were selected, excluding third-party lists of products and educational content. In the event this process led to a specific product, we navigated to the overall category page for hemp-derived Δ9-THC products. We also searched Reddit, Instagram, and YouTube to identify brands that were missed by the Google search. Companies listed on CBD Oracle’s internal database of hemp companies were also manually searched for hemp-derived Δ9-THC products. In total, we identified 89 brands currently selling hemp-derived Δ9-THC products.
We estimated that this represents around 75% of the total hemp-derived Δ9-THC market, as of April 2022. It is unlikely that the search strategy identified all brands, particularly local brands or those with no online presence. It was estimated, as a result of this and our overall familiarity with the market, that the strategy captured around 75% of the market. This estimate is imperfect by definition, because it cannot be precisely known how many brands exist beyond the boundaries of our online search. With this in mind, we estimate that there were 120 brands selling hemp-derived Δ9-THC products as of April 2022.
To select specific products for the lab analysis, companies with a TrustPilot rating lower than "4" or with a Better Business Bureau (BBB) grade below "B" were excluded, as were any companies which didn’t ship to California and any products that didn’t mention a specific dosage of THC. Companies were also ranked for popularity using the number of customer reviews on-site and followings on social media websites. We selected the Δ9-THC product from each company with the highest number of customer reviews. In some cases, we bought multiple products from the same manufacturer to cover more types of product.
We ordered a total of 53 products with a credit card and had them shipped to the CBD Oracle office in Tustin, CA. However, owing to the nature of the market, the majority of them were edibles. The products included gummies (38 products), tinctures (3), vape pens (1), cookies (2), brownies (1), chocolate (1), candies (3), beverages (3), and rice krispies (1). The vast majority sold some form of edibles (total 46), but we identified a few companies offering tinctures, some offering beverages, and one that offers a vape pen. The 53 products came from 48 different brands, which we estimate represented 40% of the total hemp-derived Δ9-THC product market, as of April 2022. Manufacturers came from multiple states: AZ, CA, CO, FL, GA, IN, MA, MI, MN, MO, NC, NJ, NV, NY, OR, TN, TX, and WI.
Lab analysis
Products were collected for lab analysis directly from the office in their original, sealed packaging and were tested within two weeks of purchase to avoid degradation. The lab analyses were performed by InfiniteCAL, a California Department of Cannabis Control (DCC)- and International Organization for Standardization/International Electrotechnical Commission (ISO/IEC 17025)-accredited lab. All products were tested for potency, and 10 randomly selected products were tested for impurities, including pesticides, mycotoxins, residual solvents, microbial contamination, heavy metals, and foreign materials.
Potency analyses for the mass/mass percentage concentrations of cannabinoids (Δ9-THC, Δ8-THC, CBD, tetrahydrocannabinolic acid [THCA], cannabidiolic acid [CBDA], cannabigerol [CBG], cannabigerolic acid [CBGA], cannabinol [CBN], tetrahydrocannabivarin [THCV], cannabidivarin [CBDV], and cannabichromene [CBC]) were performed using ultra-high-performance liquid chromatography coupled with a diode array detector (UHPLC-DAD), and concentrations are determined in relation to a calibration curve established based on certified reference materials.
Δ9-THC was extracted from the gummies/edibles using one of two parallel validated procedures. The standard procedure is as follows: Solid edible samples are cryoground to a fine powder to ensure homogeneity. A subsample (3.0 g) is weighed in a 50-mL centrifuge tube containing steel balls. Forty milliliters of methanol is added to the tube and subsequently weighed to determine the exact volume of solution. Solutions are then sonicated in a 55 °C water bath then vortexed. Solid edibles should be reduced to a silt consistency, and therefore, it may be necessary to repeat and alternate sonication and vortexing steps. After the desired consistency is reached, samples are centrifuged for three minutes at 4200 rpm. A subsequent dilution step is performed with methanol in a separate 15-mL tube. Solutions are then filtered with a 0.22-um filter into a 2-mL autosampler vial.
For samples suspected or confirmed to contain gelatin (which includes samples that do not reach the desired consistency using the standard procedure), an alternate similar procedure mirrors the standard preparation with the following changes: a mixture of 50:50 water/methanol is used in place of methanol for the extraction step, and the dilution step uses acetonitrile instead of methanol and is also centrifuged for three minutes at 4200 rpm.
The measured potency depends on how well the THC was extracted from the products. However, InfiniteCAL has performed extensive validation on edible products, with both internal sample recovery experiments (using edibles spiked with known amounts of cannabinoids) as well as external blind proficiency tests, which have shown the extraction and analysis techniques used to be both thorough and robust. Validation data can be provided upon request.
Pesticide and mycotoxin levels were determined using a combination of gas chromatography triple quad mass spectrometry (GC MS/MS) and liquid chromatography triple quad mass spectrometry (LC-MS/MS). Concentrations of arsenic (As), cadmium (Cd), mercury (Hg), and lead (Pb) were determined using inductively coupled plasma mass spectrometry (ICP-MS). Analyses for heavy metals were conducted in kinetic energy discrimination (KED) mode, using helium (He) as the collision gas and argon (Ar) as the carrier gas.
Residual solvent and terpene analyses were performed using headspace gas chromatography single quad mass spectrometry (HS-GC-MS). Microbial analysis was performed using real-time polymerase chain reaction (qPCR), with aliquots taken from the batch being incubated for 24 hours to allow microbial growth before testing. Moisture content was determined using a moisture analyzer, with loss of moisture from a pre-defined sample calculated gravimetrically. Finally, foreign material testing was performed visually, either unaided or with a microscope magnifier.
Full details of the methodology are available from InfiniteCAL.[17]
Δ9-THC conversion markers
Samples were analyzed to determine whether the Δ9-THC present was naturally occurring in the hemp plant or whether it was produced through conversion from CBD. While it is not possible to determine the source of the Δ9-THC with absolute certainty, there are several indicators that strongly suggest one of three sources: the Δ9-THC naturally produced by a hemp plant, Δ9-THC sourced from a cannabis plant, and Δ9-THC resulting from a conversion from CBD. Additional File 1 contains more detail about the markers used and the underlying chemistry. Note that these analyses were only performed with 49/53 products, due to very low quantities of minor cannabinoids in three samples, which made identification of the source challenging, and the remaining sample contained no THC.
Trans:cis-Δ9-THC ratio
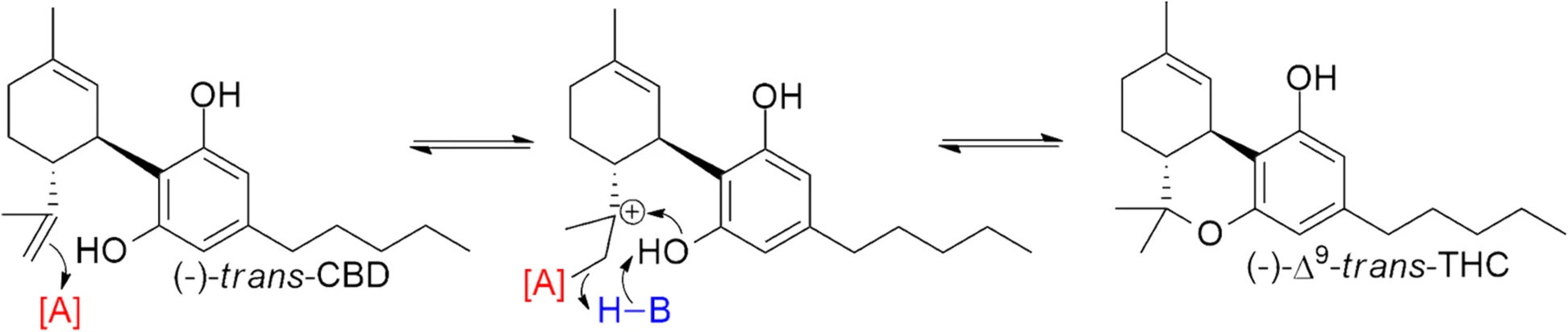
Schafroth et al.[18] found that while cis-Δ9-THC was entirely absent from a high-THC Bedrocan cultivar, 28/31 (90.3%) of hemp plants had trans:cis ratios between 1.3:1 and 8:1. These observations suggest that the biosynthetic pathways to produce Δ9-THC in classical hemp strains are not stereospecific and produce both trans-Δ9- and cis-Δ9-THC, while high-THC cannabis strains have a strongly stereospecific pathway to produce the (-)-trans-Δ9-THC. The delineation of the two strain types based on the presence/absence of cis-Δ9-THC can therefore provide potential markers to identify the source of Δ9-THC.
Since it is possible to synthetically form (-)-trans-Δ9-THC directly from (-)-trans-CBD through an isomerization-free mechanism (Fig. 1), the ratios of trans:cis-Δ9-THC in distillate converted from CBD can be expected to far exceed the ratios seen in natural hemp extracts. In contrast, oxidative cyclization from CBGA is the main source for biosynthesized THCA[19], and while natural conversion from CBD could theoretically produce cis-Δ9-THC, this process would also lead to substantial amounts of Δ8- and Δ10-THC in Cannabis plants[20], which is not observed. Therefore, in this analysis, trans:cis ratios > 8:1 are taken as evidence that the source of the THC is not hemp. If there is no cis-Δ9-THC in the sample, it is likely the THC is sourced from cannabis.
|
The quantity of Δ8-THC and Δ8-iso-THC
Δ8-THC occurs naturally in Cannabis sativa L., but only in negligible amounts.[8] Δ8-THC is formed during conversion from CBD to Δ9-THC[10], as is Δ8-iso-THC (along with its isomerized partner Δ4(8)-iso-THC). This “miscyclization” only presents itself in conversion reactions. This means that products created using naturally sourced Δ9-THC should have little to no Δ8-THC, with >1% Δ8-iso-THC+Δ8-THC (relative to the Δ9-THC amount) being taken as evidence of conversion from CBD via cyclization.[10]
Delineation of the Δ8-iso-THC and Δ8-THC amounts was not performed for this study, as the samples were run under standard UHPLC-DAD conditions for quantitation. The reported amounts of Δ8-THC, therefore, represent the combined contributions of both compounds.
The quantity of cannabigerol
The presence and quantity of CBG and other minor cannabinoids were also used as indicators of the source of the Δ9-THC. Just as the Δ8-THC in commercial products is produced via cyclization from CBD[8] because levels in “hemp” (i.e., Cannabis sativa L. plants with less than 0.3% Δ9-THC) are not naturally high enough to have a psychoactive effect, the low Δ9-THC levels in many hemp plants[18][21][22] may encourage manufacturers to use the same approach. Since CBD is the starting point for the cyclization reactions, the most efficient starting material is high-purity CBD “isolate,” which is not likely to have significant amounts of CBG present.
However, while natural hemp[18][21] and cannabis[23] contain minor cannabinoids such as CBG, this is not a product of the cyclization reaction.[8] If CBG is not present in the starting material nor a product of the conversion, it would not be expected to be present in converted Δ9-THC. Therefore, products with low quantities of CBG (<1% of total Δ9-THC content) are more likely to use converted Δ9-THC and those with higher quantities are more likely to use naturally sourced Δ9-THC.
Exact translation of fixed metrics for the Δ9-THC products in the study was not possible due to the wide range of Δ9-THC quantity in each product, but using the relative amounts of the three components along with some judgment calls allowed for grouping of each product into the three categories with reasonable confidence.
Age verification checks
Since all products were purchased from the companies’ websites, their use of age verification measures was considered. For each product, we noted if they required an ID to be presented on purchase or if an easily-circumvented method (e.g., simply entering a birth date)[24] was used. Additionally, we also noted how many products required an adult signature on delivery.
Packaging and labeling
The 53 products considered were inspected for warning labels, batch IDs, child-resistant containers, and the cannabis universal symbol (intended to alert consumers that the product contains large amounts of THC).
We define a warning label as a clear statement on the packaging that the product is intoxicating, is dangerous to minors and pets, or has specific situations in which you should not use the product, such as:
- For adults 18+, or 21+ where state law applies
- Will intoxicate, use extreme caution
- Keep away from children or pets
- Don’t drive after using
- Don’t operate heavy machinery
- Don’t consume if pregnant or breastfeeding
- Don’t consume if you are subject to drug testing
- Consult with your physician before use
Batch IDs are unique codes which identify a specific production batch of a product, thus enabling identification of other affected products in the event of contamination. These can have numerous formats, with an example from a purchased product being “E21362-10HC.”
Child-resistant containers are defined in law[25] as those which 85% of children aged three to five cannot open within five minutes without a demonstration and which 80% still cannot open even after a demonstration. For example, a child-resistant cap may require the user to push down and twist the cap to open, rather than simply twisting. We did not test the packaging first-hand or verify that it met the legal definition, but took the presence of child-proofing mechanisms (such as a child-resistant cap) as evidence of a child-resistant container.
The cannabis universal symbol (Fig. 2) or some variation thereof is used to signify to consumers that the product contains cannabis and may be intoxicating. This is not required under the 2018 Farm Bill[5], but it is required for high-THC products sold in adult-use markets such as California.[26]
|
Lab reports provided by companies
In most cases, hemp consumers must depend on a certificate of analysis (COA) provided by the company itself to determine the true potency and safety of the product in question. These were also analyzed, in particular, whether they were tested for impurities or just potency, whether the lab used was ISO-accredited, and whether they were DEA-certified.
Results
Advertised and measured Δ9-THC potencies vs. adult-use states’ standard dosage
The considered products advertised between 0.5 and 40 mg of Δ9-THC per serving, with an average of 12.98 mg per serving across all products. Even the average figure is higher than the limit of 10 mg Δ9-THC placed on edibles in legal states such as Colorado and California, and the highest exceeds it by a factor of four. The mode of the dataset was 10 mg, but many products go beyond this limit. Based on lab-measured potencies, the mean was 10.08 mg, which is still slightly above the limit for most legal states. The highest lab-measured potency was 36.68 mg/serving, almost 3.7-fold higher than most adult-use limits.
Potencies of other cannabinoids
The products sampled also contained small amounts of other cannabinoids. On average, the products contained 0.88% CBD, 0.026% CBC, 0.024% CBG, 0.004% CBN, and 0.03% Δ8-THC.
Legality of commercial hemp Δ9-THC products
Industrial hemp products are considered legal if they contain less than 0.3% Δ9-THC by dry weight. Of the 53 products analyzed, 96.2% (51 products) fell within the legal limit for Δ9-THC. The 2 products that exceeded the limit were the Blueberry Citrus Burst gummies from Delta Extrax (0.419% Δ9-THC) and the Straw Blasted gummies from Hixotic (0.31% Δ9-THC). The average for all products was 0.154% Δ9-THC, showing clearly that in many cases, the products are not even close to the legal limit. Overall, the vast majority of the hemp-derived Δ9-THC products likely fall within limits specified by the Farm Bill.
Comparison of advertised and measured Δ9-THC potency
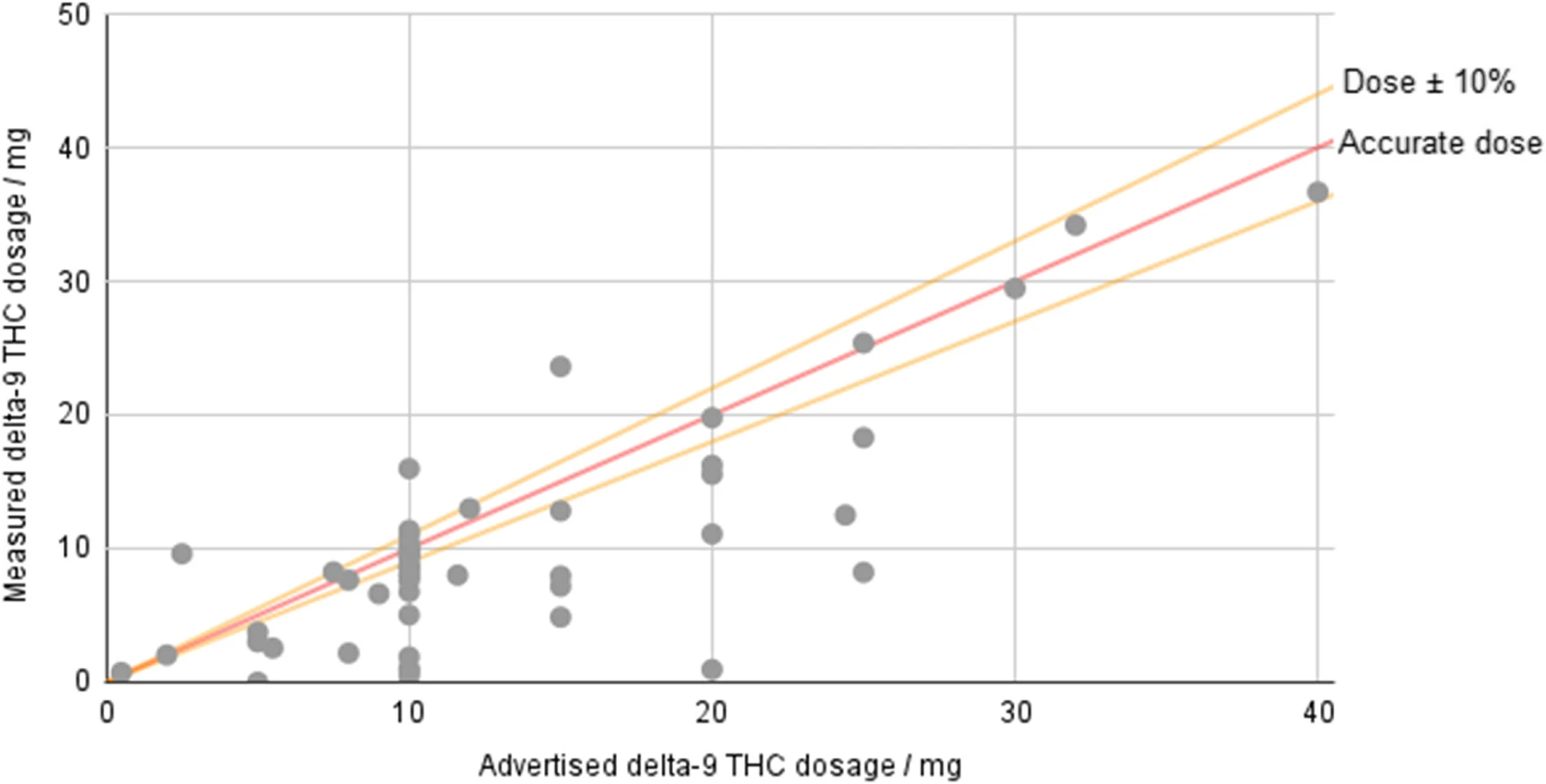
The measured amounts of Δ9-THC were compared with the amounts listed on the website and/or product packaging. Considering anything within 10% of the stated potency to be “accurately labeled,” the results show that 66.0% (35/53) of products were not accurately labeled. For all of the products, the average measured potency was 82.4% of the advertised amount (i.e., the mean of [measured potency/advertised potency] × 100 % = 82.4%). For the inaccurately labeled products, the average was 73.3% of the advertised amount. Overall, 88.6% of the mislabeled products contained less Δ9-THC than advertised (Fig. 3).
|
Impurities in Δ9-THC products
No pesticide residue, solvent residue, heavy metal contamination, microbial contamination, mycotoxins, or foreign matter were detected in any of the 10 products that were tested.
Trans:cis-Δ9-THC ratios
The ratio of trans to cis Δ9-THC was used to determine whether the product likely used THC either sourced from cannabis or converted via cyclization, with hemp plants generally having a ratio of 8:1 or lower.[18] The analysis showed that 77.6% (38/49) of products had ratios above the range expected for hemp plants.
Δ8-THC + Δ8-iso-THC content of products
With only negligible quantities of Δ8-THC in natural hemp or cannabis plants, products with Δ8-THC + Δ8-iso-THC at levels >1% of the total Δ9-THC content likely involved some THC production from cyclization. A total 63.3% (31/49) of products contained more than 1% Δ8-THC + Δ8-iso-THC.
CBG content of products
Minor cannabinoids such as CBG are commonly found in products sourced from hemp or cannabis, but are almost entirely absent from products produced via cyclization. In the analysis, 38.8% (19/49) of products contained less than 1% CBG (relative to total Δ9-THC content) and thus were less likely to be made using natural cannabis or hemp extract.
Naturally occurring vs. converted Δ9-THC
Based on the lab analysis and specifically the factors discussed above, InfiniteCAL estimated that 49.0% (24/49) of products used Δ9-THC that had been converted from CBD through cyclization. The results also suggested that 26.5% (13/49) used Δ9-THC sourced from cannabis to meet their stated dosage and that only 18.4% (9/49) probably used natural hemp-derived Δ9-THC. The remaining four products studied could not be classified.
Analyzing the COAs provided by hemp-derived Δ9-THC companies
84.9% (45/53) of the hemp-derived Δ9-THC products investigated had an associated COA available to customers. Of these products with COAs, 71.1% (32/45) were not tested for impurities, with tests conducted only to verify potency. In total, then, 75.5% (40/53) of products were not proven to be free from impurities (although testing a random sample of 10 revealed no issues, as discussed above).
80% (36/45) of COAs were obtained from ISO/IEC 17025:2017-certified labs, and accreditation certificates were verified through Perry Johnson Laboratory Accreditation (PJLA), American Association for Laboratory Accreditation (A2LA), and International Laboratory Accreditation Cooperation (ILAC) databases. In addition, 51.1% (23/45) of COAs were from labs that were DEA-certified.
Labeling of hemp-derived Δ9-THC products
Customers depend on labels for important information and warnings about the contents of a product. It was found that 83.0% (44/53) of products had some form of warning label.
However, 73.6% (39/53) of products did not include the cannabis universal symbol (or some variation thereof) on their packaging. In adult-use states, this is required to inform customers that the product contains large amounts of THC.[26] Additionally, 50.9% (27/53) of products did not include a batch ID on the label, which means it will be difficult or even impossible to trace any issues back to a specific batch and inform consumers and retailers of the issue.
Age verification and child-resistant packaging for hemp-derived Δ9-THC products
The products we sampled largely did not include child-resistant packaging, with 81.1% (43/53) not using a child-resistant container. This would be illegal in adult-use markets such as California[26], for instance.
For age verification, 84.9% (45/53) of companies did not perform online age verification at checkout. The companies which did all used AgeChecker, which verifies age by cell phone verification and requiring customers to upload a “selfie” with their ID, as well as provide a clear picture of it. Additionally, 98.1% of companies (52/53) did not require an adult signature on delivery. All but one product was simply left in the mailbox without obtaining any form of signature.
Discussion
The lab analysis revealed that the industry offers what it claims in some ways, but potentially misleads or does not inform customers in others. On the one hand, it is clear that the vast majority of products (96.2%) fall within the legal Δ9-THC limits established by the 2018 federal Farm Bill.[5] Additionally, the tests conducted suggest that impurities are not a substantial issue for companies producing hemp-derived Δ9-THC products.
However, the investigation also revealed several points which may be concerning from a consumer perspective. Firstly, in many cases, the advertised dosages are substantially higher than would be allowed in any regulated, legal, high-THC cannabis market in the country. Secondly, most of the products (73.6%) do not include the cannabis universal symbol to warn potential consumers of their high Δ9-THC content. Despite the fact that most (84.9%) products are backed with a COA, since most of these do not include impurity testing, 75.5% of hemp-derived Δ9-THC products are not safety tested. This problem is compounded by the lack of batch labeling for 50.9% of products. Finally, and potentially the largest issue for consumers is the fact that 66.0% of products differ from the Δ9-THC dosage stated on their labels by 10% or more, usually providing less than advertised.
Considering the potential risks of excessive amounts of THC[27], the combination of factors here could be a cause for concern. Customers in adult-use states expect the cannabis universal symbol or some variation thereof on high-THC products, with over 80% of states with medical cannabis requiring this, for instance.[28] In many cases, even the advertised dosages exceed those considered acceptable in adult-use states. Additionally, two out of three products differ from the stated dose by over 10%, which makes it difficult—if not impossible—for customers to know how much THC they will actually consume. The problem from a consumer perspective is one of consent: people may buy under the assumption that the product is “just hemp” or at least "not high-THC," and others may get more THC than they wanted or were informed of through labeling. These issues could be solved by the industry, but legislators might also take steps such as setting a total THC cap on hemp products or requiring accurate labeling.
Despite some concern about youth access to intoxicating hemp products[29][30], the analysis revealed many problems with how companies attempt to prevent under-age purchases and access. In particular, only eight of 53 products (15.1%) required any form of age verification prior to the order being placed. This means that in the remainder of cases, all people had to do prior to making a purchase is either input a date of birth or click a button confirming that he/she is over 21. These systems can be easily circumvented or simply lied to[24] and so do not constitute age verification. The remaining purchases had age verification through AgeChecker, which uses public records and images of ID to determine the customer’s true age. Additionally, only one product (1.9%) required an adult signature on delivery, and the remainder simply allowed the delivery to be left in the mailbox.
While getting a valid card to pay may be a challenge, this is essentially the only thing preventing youths from accessing high-THC hemp products such as those in this study. From this point onwards, a youth could easily place an order (simply using an alternative website if they initially chose one of the 15% which performs age checks) and receive the delivery without having to present ID at any stage. In the absence of further laws—requiring a signature on delivery for intoxicating hemp products, for instance—this situation is difficult to rectify.
Finally, the lab analysis showed that 96.2% of hemp-derived Δ9-THC products do fall within the limits established by the Farm Bill. This is a positive sign for the industry, noting that current legal opinion suggests that hemp-derived Δ9-THC is legal in the absence of further state legislation.
However, the finding that 49% of products used Δ9-THC converted from CBD may challenge this in some localities. Colorado[11], for example, restricts Δ8-THC on the basis that “chemically modifying or converting any naturally occurring cannabinoids from industrial hemp is non-compliant with the statutory definition of ‘industrial hemp product'," and Massachusetts[12] has a similar approach. These laws would also apply to Δ9-THC products advertised as hemp if levels were increased by conversion from CBD. Maryland is also considering[31] regulations on THC products that are synthetically or artificially derived.
Limitations and implications of the research
There are some limitations of the analysis. Firstly, owing to the nature of the market, the majority of products (86.8%) tested were gummies, candies, or other edibles, with relatively few drinks, tinctures, and vaping products. While this represents the market closely, it may be that other product types differ in some important ways (for example, being more likely to exceed the legal limit for Δ9-THC ). Unfortunately, the sample did not include enough non-edible samples to investigate differences by product type. Only one sample of each product was analyzed, but values could (and likely do) vary by the individual sample and not just by product. There was also some time (less than two weeks in all cases) between purchase and testing, which could feasibly have impacted the results through degradation, despite all products being sealed until testing.
The results show the consequences of the legal loophole which hemp-derived Δ9-THC companies are currently operating in. With no centralized regulatory body, and very little in the way of state-specific regulations in most cases, intoxicating hemp products are currently allowed to operate with minimal oversight. This is why, for instance, that not all products are accompanied by a COA, why dosages (both advertised and actual) vary so wildly, and why in most cases age verification is essentially absent.
There are three avenues that could help companies and regulators find a potential solution to these issues. Firstly, states with adult-use or medical marijuana programs could incorporate intoxicating hemp products into their existing legal framework for legal cannabis. This would include, for instance, maximum doses of Δ9-THC (or indeed other THCs) per serving and per package, as well as lab testing requirements. Secondly, states could improve their hemp legislation to account for intoxicating products, enabling them to implement similar restrictions on THC-rich products and require age verification. Finally, the industry itself could adopt reasonable standards in the absence of official guidance. In the manner most companies now offer COAs for their products, they may (through hemp industry organizations) institute mandatory age checks and standardized testing requirements.
Conclusion
The legal status of hemp-derived Δ9-THC products in America essentially permits their open sale while placing very few requirements on the companies selling them. The results of this lab and market analysis show the consequences of this policy: products are sold that have 3.7 times the THC content of edibles in adult-use states, and age verification, safety testing, and accurate dosages are neither required nor often present. On top of this, 49% of products use THC converted from CBD, which explicitly contradicts the law in some states but raises issues with the Farm Bill’s definition of “hemp” in any case. The industry meets some common-sense requirements—such as almost all products being within legal limits and none having issues with impurities—but there is also a lot of work to do in several key areas.
Abbreviations, acronyms, and initialisms
- CBD: cannabidiol
- CBDA: cannabidiolic acid
- CBDV: cannabidivarin
- CBG: cannabigerol
- CBGA: cannabigerolic acid
- CBN: cannabinol
- COA: certificate of Analysis
- DCC: Department of Cannabis Control
- DEA: Drug Enforcement Administration
- GC MS/MS: gas chromatography triple quad mass spectrometry
- HPLC: high-performance liquid chromatography
- HS-GC-MS: headspace gas chromatography single quad mass spectrometry
- ICP-MS: inductively coupled plasma mass spectrometry
- ISO/IEC: International Organization for Standardization / International Electrotechnical Commission
- KED: kinetic energy discrimination
- LC-MS/MS: liquid chromatography triple quad mass spectrometry
- qPCR: real-time polymerase chain reaction
- THC:: tetrahydrocannabinol
- THCA: tetrahydrocannabinolic acid
- THCV: tetrahydrocannabivarin
- UHPLC-DAD: ultra-high-performance liquid chromatography coupled with a diode array detector
- UV: ultraviolet
Supplementary information
- Additional File 1: Kramer, M.; Lomas, S., 2017 (.docx)
Acknowledgements
The authors would like to acknowledge Houman Shahi, for his support, guidance, and organizational assistance throughout the process.
Author contributions
LJ and MM planned the research and wrote the majority of the manuscript. DB planned and organized the data collection. EP, JS, DM, and SA performed the lab work and wrote the “∆9 THC Conversion Markers” supplementary information, with support from DB. All authors approved the finished version of the manuscript.
Funding
The research was funded by CBD Oracle.
Availability of data and materials
The original lab reports for each product are available in a Google Drive.
Competing interests
LJ and MM are employed by CBD Oracle, a CBD and cannabis consumer website. While they have no financial incentive for any particular result, they are advocates of cannabis and hemp. The remaining authors declare that they have no competing interests.
References
- ↑ Cooper, Ziva D.; Haney, Margaret (1 January 2009). "Actions of delta-9-tetrahydrocannabinol in cannabis: Relation to use, abuse, dependence" (in en). International Review of Psychiatry 21 (2): 104–112. doi:10.1080/09540260902782752. ISSN 0954-0261. PMC PMC2731700. PMID 19367504. http://www.tandfonline.com/doi/full/10.1080/09540260902782752.
- ↑ Russo, Ethan B.; McPartland, John M. (1 February 2003). "Cannabis is more than simply Δ9-tetrahydrocannabinol" (in en). Psychopharmacology 165 (4): 431–432. doi:10.1007/s00213-002-1348-z. ISSN 0033-3158. http://link.springer.com/10.1007/s00213-002-1348-z.
- ↑ Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda; Board on Population Health and Public Health Practice; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine (31 March 2017). "Chapter 4: Therapeutic Effects of Cannabis and Cannabinoids". The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, D.C.: National Academies Press. doi:10.17226/24625. ISBN 978-0-309-45304-2. https://www.nap.edu/catalog/24625.
- ↑ National Conference of State Legislatures (2023). "Report: State Medical Cannabis Laws". National Conference of State Legislatures. https://www.ncsl.org/health/state-medical-cannabis-laws.
- ↑ 5.0 5.1 5.2 "Agriculture Improvement Act of 2018" (PDF). U.S. Government Publishing Office. 3 January 2018. https://www.congress.gov/115/bills/hr2/BILLS-115hr2enr.pdf.
- ↑ 6.0 6.1 Johnson-Arbor, Kelly; Smolinske, Susan (1 June 2022). "The current state of delta-8 THC" (in en). The American Journal of Emergency Medicine 56: 259–261. doi:10.1016/j.ajem.2021.06.066. https://linkinghub.elsevier.com/retrieve/pii/S0735675721005520.
- ↑ Kruger, Jessica S.; Kruger, Daniel J. (1 December 2022). "Delta-8-THC: Delta-9-THC’s nicer younger sibling?" (in en). Journal of Cannabis Research 4 (1): 4. doi:10.1186/s42238-021-00115-8. ISSN 2522-5782. PMC PMC8725316. PMID 34980292. https://jcannabisresearch.biomedcentral.com/articles/10.1186/s42238-021-00115-8.
- ↑ 8.0 8.1 8.2 8.3 8.4 Tagen, Michael; Klumpers, Linda E. (1 August 2022). "Review of delta‐8‐tetrahydrocannabinol (Δ 8 ‐THC): Comparative pharmacology with Δ 9 ‐THC" (in en). British Journal of Pharmacology 179 (15): 3915–3933. doi:10.1111/bph.15865. ISSN 0007-1188. https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bph.15865.
- ↑ Casati, Sara; Rota, Paola; Bergamaschi, Roberta F.; Palmisano, Erika; La Rocca, Paolo; Ravelli, Alessandro; Angeli, Ilaria; Minoli, Mauro et al. (23 November 2022). "Hexahydrocannabinol on the Light Cannabis Market: The Latest “New” Entry" (in en). Cannabis and Cannabinoid Research: can.2022.0253. doi:10.1089/can.2022.0253. ISSN 2578-5125. https://www.liebertpub.com/doi/10.1089/can.2022.0253.
- ↑ 10.0 10.1 10.2 Marzullo, Paola; Foschi, Francesca; Coppini, Davide Andrea; Fanchini, Fabiola; Magnani, Lucia; Rusconi, Selina; Luzzani, Marcello; Passarella, Daniele (23 October 2020). "Cannabidiol as the Substrate in Acid-Catalyzed Intramolecular Cyclization" (in en). Journal of Natural Products 83 (10): 2894–2901. doi:10.1021/acs.jnatprod.0c00436. ISSN 0163-3864. PMC PMC8011986. PMID 32991167. https://pubs.acs.org/doi/10.1021/acs.jnatprod.0c00436.
- ↑ 11.0 11.1 Colorado Department of Public Health & Environment (14 May 2021). "Re: Production and/or Use of Chemically Modified or Converted Industrial Hemp Cannabinoids" (PDF). Colorado Department of Public Health & Environment. https://www.denvergov.org/files/assets/public/v/1/public-health-and-environment/documents/phi/dehs_mfdfd_industrialhemp_delta8_notice_cdphe_logo_051421.pdf.
- ↑ 12.0 12.1 Massachusetts Department of Agriculture (2023). "Hemp in Massachusetts: FAQs". Commonwealth of Massachusetts. https://www.mass.gov/guides/hemp-in-massachusetts-faqs#-is-it-legal-to-manufacture-delta-8-thc-from-hemp. Retrieved 17 June 2023.
- ↑ Brangham, W. (1 August 2014). "Edible marijuana rules tightened in Colorado". PBS News Hour. NewsHour Productions, LLC. https://www.pbs.org/newshour/nation/edible-marijuana-rules-tightened-colorado.
- ↑ Romine, S. (8 July 2019). "Before you buy cannabis, brush up on California laws and safety precautions". USA Today. Gannett Satellite Information Network, LLC. https://www.usatoday.com/story/sponsor-story/higher-elevation/2019/07/08/before-you-buy-cannabis-brush-up-california-laws-and-safety-precautions/1672816001/.
- ↑ Department of Cannabis Control (September 2023). "Medicinal and Adult-Use Commercial Cannabis Regulations" (PDF). State of California. https://cannabis.ca.gov/wp-content/uploads/sites/2/2023/09/dcc_commercial_cannabis_regulations-1.pdf.
- ↑ U.S. Hemp Roundtable (30 April 2021). "Delta-8 THC Sales Across The States". HempSupporter.com. U.S Hemp Roundtable, Inc. https://hempsupporter.com/news/delta-8/. Retrieved 17 June 2023.
- ↑ Swider, J.; Marelius, D. (December 2022). "InfiniteCAL Certificate of Methodology" (PDF). Infinite Chemical Analysis Labs, LLC. https://infinitecal.com/wp-content/uploads/2022/12/InfiniteCAL-Certificate-of-Methodology-2022.pdf.
- ↑ 18.0 18.1 18.2 18.3 Schafroth, Michael A.; Mazzoccanti, Giulia; Reynoso-Moreno, Ines; Erni, Reto; Pollastro, Federica; Caprioglio, Diego; Botta, Bruno; Allegrone, Gianna et al. (24 September 2021). "Δ 9 - cis -Tetrahydrocannabinol: Natural Occurrence, Chirality, and Pharmacology" (in en). Journal of Natural Products 84 (9): 2502–2510. doi:10.1021/acs.jnatprod.1c00513. ISSN 0163-3864. https://pubs.acs.org/doi/10.1021/acs.jnatprod.1c00513.
- ↑ Taura, Futoshi; Sirikantaramas, Supaart; Shoyama, Yoshinari; Shoyama, Yukihiro; Morimoto, Satoshi (1 August 2007). "Phytocannabinoids in Cannabis sativa : Recent Studies on Biosynthetic Enzymes" (in en). Chemistry & Biodiversity 4 (8): 1649–1663. doi:10.1002/cbdv.200790145. ISSN 1612-1872. https://onlinelibrary.wiley.com/doi/10.1002/cbdv.200790145.
- ↑ Golombek, Patricia; Müller, Marco; Barthlott, Ines; Sproll, Constanze; Lachenmeier, Dirk W. (3 June 2020). "Conversion of Cannabidiol (CBD) into Psychotropic Cannabinoids Including Tetrahydrocannabinol (THC): A Controversy in the Scientific Literature" (in en). Toxics 8 (2): 41. doi:10.3390/toxics8020041. ISSN 2305-6304. PMC PMC7357058. PMID 32503116. https://www.mdpi.com/2305-6304/8/2/41.
- ↑ 21.0 21.1 Glivar, Taja; Eržen, Jan; Kreft, Samo; Zagožen, Marjeta; Čerenak, Andreja; Čeh, Barbara; Tavčar Benković, Eva (1 March 2020). "Cannabinoid content in industrial hemp (Cannabis sativa L.) varieties grown in Slovenia" (in en). Industrial Crops and Products 145: 112082. doi:10.1016/j.indcrop.2019.112082. https://linkinghub.elsevier.com/retrieve/pii/S0926669019310921.
- ↑ Johnson, Matthew S.; Wallace, Jason G. (7 July 2021). "Genomic and Chemical Diversity of Commercially Available High-CBD Industrial Hemp Accessions". Frontiers in Genetics 12: 682475. doi:10.3389/fgene.2021.682475. ISSN 1664-8021. PMC PMC8293613. PMID 34306025. https://www.frontiersin.org/articles/10.3389/fgene.2021.682475/full.
- ↑ Radwan, Mohamed M.; Chandra, Suman; Gul, Shahbaz; ElSohly, Mahmoud A. (8 May 2021). "Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis" (in en). Molecules 26 (9): 2774. doi:10.3390/molecules26092774. ISSN 1420-3049. PMC PMC8125862. PMID 34066753. https://www.mdpi.com/1420-3049/26/9/2774.
- ↑ 24.0 24.1 Williams, Rebecca S.; Phillips-Weiner, K. Jean; Vincus, Amy A. (1 March 2020). "Age Verification and Online Sales of Little Cigars and Cigarillos to Minors" (in en). Tobacco Regulatory Science 6 (2): 152–163. doi:10.18001/TRS.6.2.6. ISSN 2333-9748. PMC PMC7416878. PMID 32789154. https://www.ingentaconnect.com/content/10.18001/TRS.6.2.6.
- ↑ Bakshi, Arjun; Patel, Preeti (2023), "Poison Prevention Packaging Act", StatPearls (Treasure Island (FL): StatPearls Publishing), PMID 34283507, http://www.ncbi.nlm.nih.gov/books/NBK572141/
- ↑ 26.0 26.1 26.2 California Department of Cannabis Control (2023). "Requirements for cannabis goods". State of California. https://cannabis.ca.gov/licensees/requirements-cannabis-goods/.
- ↑ D’Souza, Deepak Cyril; Sewell, Richard Andrew; Ranganathan, Mohini (1 October 2009). "Cannabis and psychosis/schizophrenia: human studies" (in en). European Archives of Psychiatry and Clinical Neuroscience 259 (7): 413–431. doi:10.1007/s00406-009-0024-2. ISSN 0940-1334. PMC PMC2864503. PMID 19609589. http://link.springer.com/10.1007/s00406-009-0024-2.
- ↑ Kruger, Daniel J.; Korach, Natalie J.; Kruger, Jessica S. (1 April 2022). "Requirements for Cannabis Product Labeling by U.S. State" (in en). Cannabis and Cannabinoid Research 7 (2): 156–160. doi:10.1089/can.2020.0079. ISSN 2578-5125. PMC PMC9070747. PMID 33998880. https://www.liebertpub.com/doi/10.1089/can.2020.0079.
- ↑ Akingbasote, James; Szlapinski, Sandra; Charrette, Andrew; Hilmas, Corey J.; Guthrie, Najla (2022), "Safety of cannabis- and hemp-derived constituents in reproduction and development" (in en), Reproductive and Developmental Toxicology (Elsevier): 455–487, doi:10.1016/b978-0-323-89773-0.00024-2, ISBN 978-0-323-89773-0, https://linkinghub.elsevier.com/retrieve/pii/B9780323897730000242
- ↑ U.S. Food and Drug Administration (16 June 2022). "FDA Warns Consumers About the Accidental Ingestion by Children of Food Products Containing THC". U.S. Food and Drug Administration. https://www.fda.gov/food/alerts-advisories-safety-information/fda-warns-consumers-about-accidental-ingestion-children-food-products-containing-thc.
- ↑ Feldman, B.J. (8 July 2022). "Cannabis - Regulation - Delta-8- and Delta-10-Tetrahydrocannabinol". Maryland General Assembly. https://mgaleg.maryland.gov/mgawebsite/Legislation/Details/SB0788?ys=2022rs.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. Several URLs listed in the original were broken; presumed-correct URLs were discovered and used for this version.