Journal:Sigma metrics as a valuable tool for effective analytical performance and quality control planning in the clinical laboratory: A retrospective study
| Full article title | Sigma metrics as a valuable tool for effective analytical performance and quality control planning in the clinical laboratory: A retrospective study |
|---|---|
| Journal | National Journal of Laboratory Medicine |
| Author(s) | Karaattuthazhathu, Anupapama R.; Sathi, P.P.; Nair, Lathi; Ramees, P.M.; Manayani, Reenu; Benny, Elizabeth; Benny, Mary |
| Author affiliation(s) | KMCT Medical College |
| Primary contact | anupamarojith at gmail dot com |
| Year published | 2023 |
| Volume and issue | 12(2) |
| Page(s) | PO14 - PO18 |
| DOI | 10.7860/NJLM/2023/60440.2714 |
| ISSN | 2277-8551 |
| Distribution license | Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International |
| Website | [https://njlm.net/article_FULLTEXT.aspx?issn=0973-709x&year=2023&month=April&volume=12&issue=2&page=PO14%20-%20PO18&id=2714 https://njlm.net/article_FULLTEXT.aspx |
| Download | https://www.jcdr.net//articles/PDF/17447/58635_CE(AD)_F(IS)_PF1(AKA_SS)_PFA(SS)_PN(SS).pdf (PDF) |
|
|
This article contains rendered mathematical formulae. You may require the TeX All the Things plugin for Chrome or the Native MathML add-on and fonts for Firefox if they don't render properly for you. |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Introduction: For the release of precise and accurate reports of routine tests, its necessary to follow a proper quality management system (QMS) in the clinical laboratory. As one of the most popular QMS tools for process improvement, Six Sigma techniques and tools have been accepted widely in the laboratory testing process. Six Sigma gives an objective assessment of analytical methods and instrumentation, measuring the outcome of a process on a scale of 0 to 6. Poor outcomes are measured in terms of defects per million opportunities (DPMO).
Aim: To do the performance assessment of each clinical laboratory analyte by Six Sigma analysis and to plan and chart out a better, customized quality control (QC) plan for each analyte, according to its own sigma value.
Materials and methods: This was a retrospective observational study, conducted from January 2022 to June 2022 in the Department of Central Laboratory, KMCT Medical College, Kozhikode, Kerala, India. The precision and accuracy of 26 parameters in both hematology and biochemistry were assessed via internal quality control (IQC) and external quality assurance (EQAS) programs and analysis, and their performance was assessed by sigma analysis.
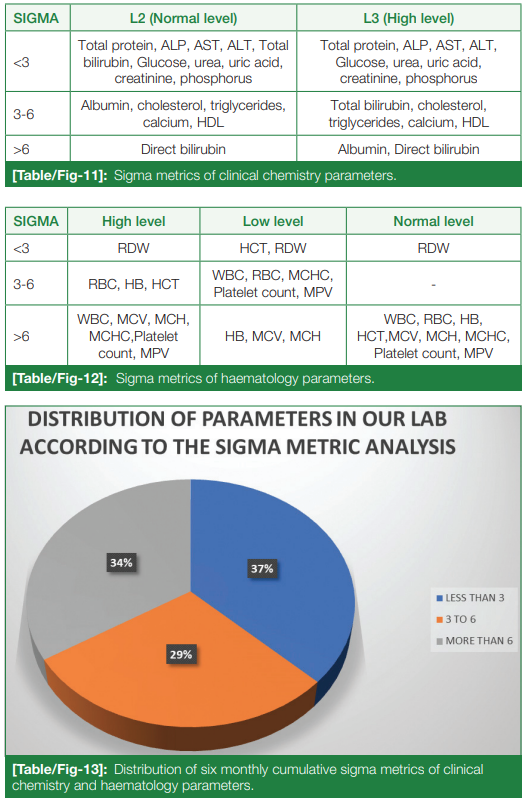
Results: Clinical chemistry parameters showed an average percentage of coefficient of variation (CV%) of 2.65% and 2.30% for all the parameters in L2 (normal level) and L3 (abnormal levels), respectively. In hematology, the average CV% came out as 1.30% (high level), 1.82% (low level), and 1.35% (normal level). These values indicate excellent precision for all parameters in both clinical chemistry and hematology, with CV% below 3.00%. It was observed in the month of May, due to reconstitution errors, that bias percent showed a setback in a few chemistry parameters, due to which the sigma was lowered. Parameters with < 3 sigma metrics (poor performance) occupy 37%, 3-6 sigma metrics (good performance) occupy 29%, and > 6 sigma metrics (world-class performance) occupy 34% of all the 26 parameters of clinical chemistry and hematology. Mean standard deviation (SD) for biochemistry parameters were calculated using the daily IQC data.
Conclusion: With the present study, sigma metric analysis provides a benchmark for the laboratory to design a protocol for IQC, address poor assay performance, and assess the efficiency of the existing laboratory processes. It is on the basis of strict QC measures and sigma analysis the Department of Central Laboratory, KMCT Medical College was able to achieve world-class performance in many analytes of clinical chemistry and hematology disciplines. However, a few analytes like alkaline phosphatase, alanine transaminase, aspartate transaminase, and total protein needed more stringent EQAS monitoring and modified QC measures.
Keywords : Six sigma, Total analytical error, Westgards rules
Introduction
In a healthcare system, laboratory diagnostics plays a very important role in accurate diagnosis and prompt disease management. The total testing process in a clinical laboratory includes pre-analytical, analytical, and post-analytical phases. Quality control (QC) measures can be applied successfully in any of the three phases. In the analytical phase, quality improvement can be done through internal quality control (IQC) and external quality assurance (EQAS) measures to ensure precision and accuracy of result reporting. IQC involves running a control sample with an identical matrix to the patient's specimen, with the sample having an established concentration range. The range should be available in high, low, or normal levels of the analyte, covering the medical decision points. EQAS or peer group programs involve periodically reporting proficiency testing samples, which are supplied by an external agency at a predefined time interval. The values obtained are compared with those obtained in other laboratories that are participating in the same program and are interpreted as SD Index or Z-score. While IQC determines the precision (expressed as a coefficient of variation or CV) of the testing process, EQAS measures its accuracy (expressed as bias). [1] The use of sigma metrics is about identifying non-conformities or errors using a technique to quantify and then minimize those defects. [2] It is the benchmarking scale where all the process defects in all three phases of a total testing process are measured and judged.
The Six Sigma method of sigma metrics was put to use decades ago, in Motorola by Sir Bill Smith, the father of Six Sigma in 1986. [3] The first paper to express its application in clinical laboratory processes was published in 2000. [4]
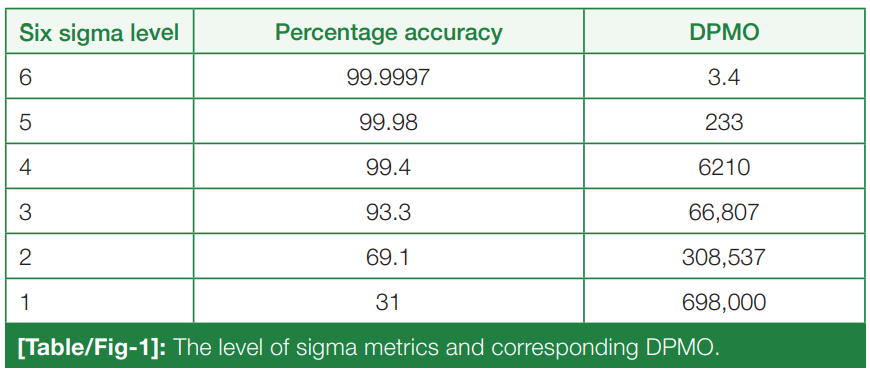
Six Sigma measures the outcomes of key process aspects, on a scale of 0 to 6. The poor outcomes are measured in terms of defects per million opportunities (DPMO). The number of defects or errors of the laboratory in any area of the total testing process can be counted or estimated and converted to the DPMO ratio. [5] The level of sigma metrics and corresponding DPMO is shown in (Table/Fig 1). [6]
|
This work has the following objectives:
• To determine the precision of 36 parameters included in the hematology and clinical chemistry sections, quantitated by percentage coefficient variation (CV%); • To assess the accuracy of the analytes, quantitated by the bias percentage of each analyte after EQAS analysis; • To determine the performance assessment of each analyte by Six Sigma analysis; and • To plan and chart out a better, customized QC plan for each analyte according to its own sigma value.
Using the sigma metrics protocol, the authors were able to effectively evaluate the analytical process control procedures in the clinical laboratory and could verify any shortcomings in the procedures. Thus, accuracy, precision, and error detection rate of the analytical phase of the total testing process may be detected and rectified.
Materials and methods
This was a retrospective observational study, conducted for a period of six months (January 2022 to June 2022) in the Department of Central Laboratory, KMCT Medical College, Kozhikode, Kerala, India. The biochemical analytes included in the study are albumin, bilirubin (total and direct), total protein, calcium, glucose, urea, creatinine, uric acid, HDL, triglycerides, cholesterol, phosphorus, aspartate transaminase, and alanine transaminase. Hematology parameters included hemoglobin (Hb), red blood cell (RBC) count, hematocrit, mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and coefficient of variation of red cell distribution width (RDW-cv), standard deviation of red cell distribution width (RDW-SD), total white blood cell (WBC) count, platelet count, and mean platelet volume (MPV).
Study procedure
The biochemistry IQCs were performed daily using L2 (normal) and L3 (pathological) levels, thrice a day. Hematological QC was performed thrice a day using three levels (High, Normal, Low).
The chemistry parameters’ QC samples were run in Randox's RX Imola and sigma were calculated on a six monthly cumulative basis. The hematology parameters’ QC samples were run in an Horiba ABX Pentra XL 80 analyzer and the CV% of each was calculated on a monthly basis, with sigma calculated on a cumulative six-month basis. In the laboratory, the IQC data of all the disciplines were interpreted daily by Levy-Jenning’s charts and Westgard’s rules. [7] Daily IQC outliers were detected and appropriate prompt corrective and preventive actions were taken. The patients’ specimens were analyzed only when the IQC results were within control limits. The authors adopted the set of Westgard’s multi-rules [7] in the laboratory as 1-2s as warning rules, and 2-2s, 1-3s, and R4s as rejection rules.
The QC practices, such as the control of material storage, reconstitution, and analysis, were done as per the manufacturer’s instructions. EQAS data was obtained by running monthly control samples from the Randox International Quality Assessment Scheme (RIQAS).[1]
Statistical analysis
Statistical analysis was performed using Microsoft Office Excel 2010. Mean standard deviation (SD) for biochemistry parameters was calculated using the daily IQC data. The CV% was calculated using the formula:
.
Bias percentage and SD index were calculated using RIQAS data for each analyte. Bias percentage was calculated using the formula:
,
where the peer group mean is the mean of all QC values of laboratories enrolled in the RIQAS program. The allowable total analytical error (TEa%) for each analyte was referenced from the Clinical Laboratory Improvement Amendments (CLIA) guidelines and the consolidated analytical performance requirements. [8] Sigma metrics for analytes were calculated using the formula:
.
Results
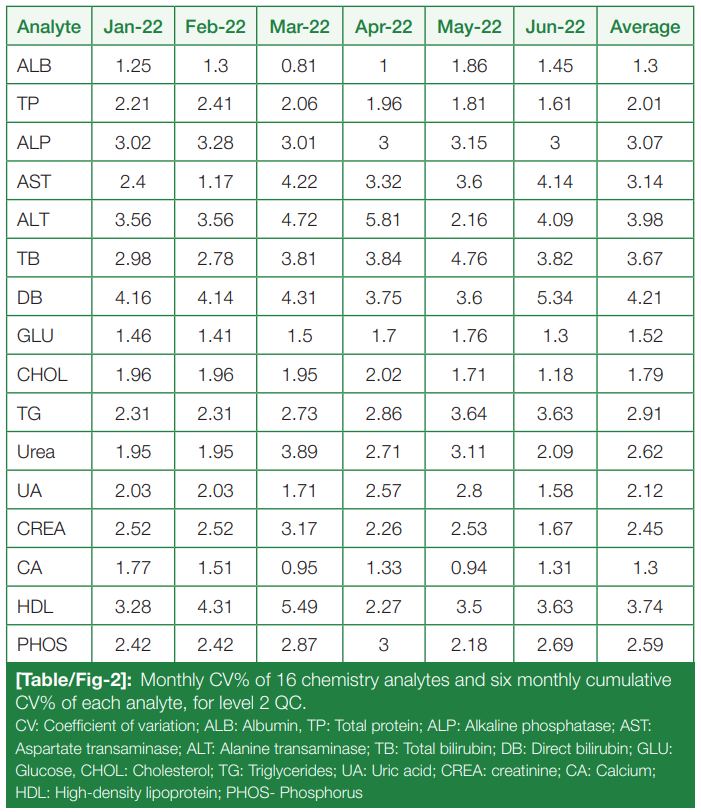
Monthly CV% of level 2 (L2) controls for the chemistry analytes, from January to June 2022, and six-month cumulative CV% for each are tabulated and summarized in (Table/Fig 2).
|
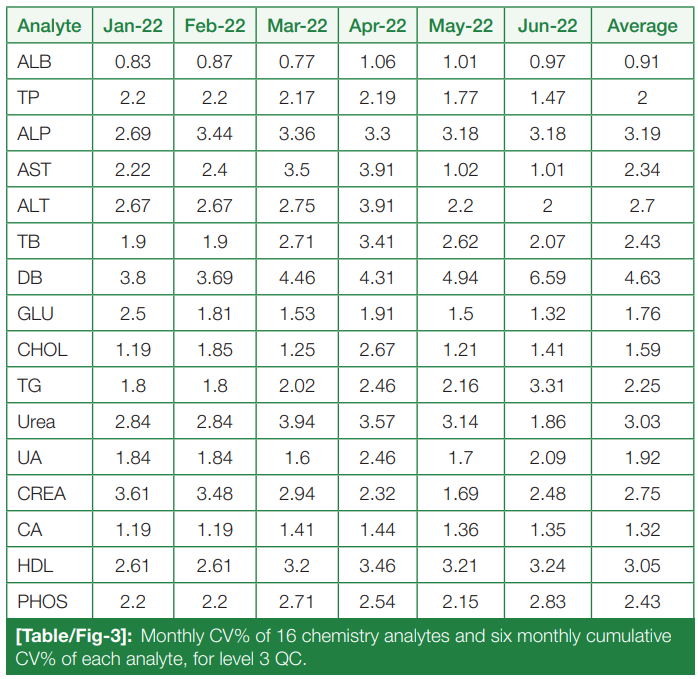
Monthly CV% of Level 3 (L3) controls for the chemistry analytes, from January to June 2022, and six-month cumulative CV% for each are tabulated and summarized in (Table/Fig 3).
|
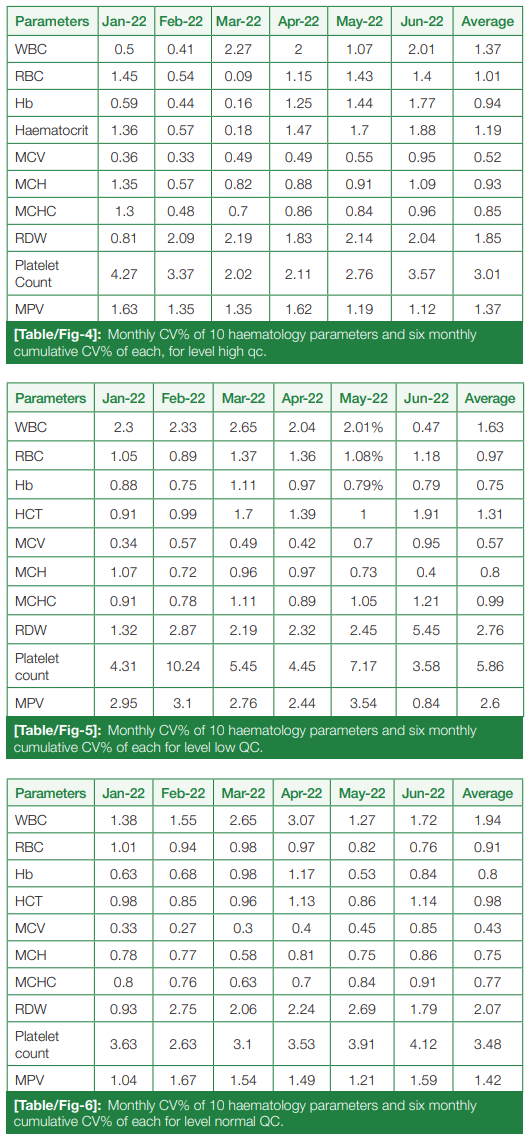
Monthly CV%, from January 2022 to June 2022, of hematological parameters for all three levels (High, Low, and Normal) were calculated, and six-month cumulative CV% were calculated for each parameter, summarized in (Table/Fig 4), (Table/Fig 5), and (Table/Fig 6), respectively.
|
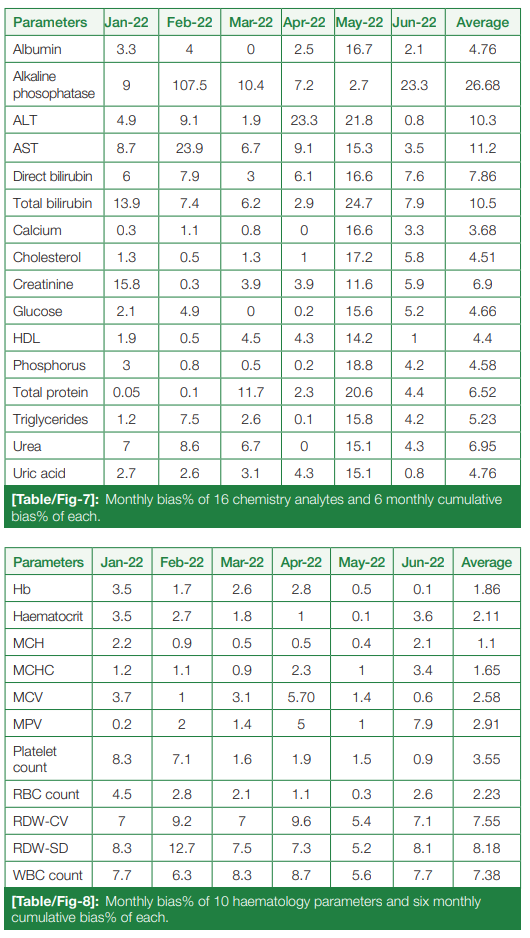
Monthly bias percentage, from January 2022 to June 2022, and six-month cumulative bias percentage of chemistry and hematological parameters were calculated, summarized in (Table/Fig 7) and (Table/Fig 8), respectively.
|
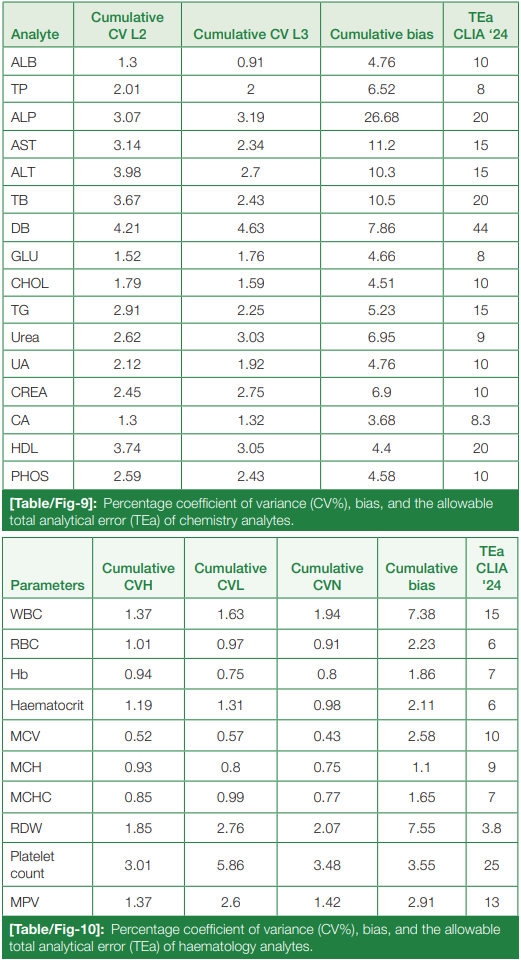
The cumulative CV% and cumulative bias percentage of each analyte in both chemistry and hematology are clubbed together, and the allowable TEa% of all the chemistry and hematological parameters according to the CLIA guidelines are summarized in (Table/Fig 9) and (Table/Fig 10), respectively.
|
Average CV% was calculated, and with the above-mentioned formula, sigma metrics was calculated for each clinical chemistry and hematology parameter, as seen in (Table/Fig 11) and (Table/Fig 12), respectively. The analytes were broadly categorized into sigma < 3 (unacceptable), sigma 3-6 (good), and sigma > 6 (excellent), using a pie diagram (Table/Fig 13). Upon analysis, parameters with < 3 sigma metrics (poor performance) occupied 37%, 3-6 sigma metrics (good performance) occupied 29%, and > 6 sigma metrics (world-class performance) occupied 34% of all the 26 parameters of clinical chemistry and hematology.
|
Acknowledgements
The authors acknowledge the laboratory technical staffs for their cooperation and assistance.
Ethics
Ethics committee approval and informed consent was obtained for this study.
Competing interests
None declared.
References
- ↑ "Randox International Quality Scheme". Randox International. November 2020. Archived from the original on 20 January 2022. https://web.archive.org/web/20220120044738/https://www.randox.com/wp-content/uploads/delightful-downloads/2020/11/LT033-RIQAS-Explained-OCT20-compressed.pdf.
Notes
This presentation is faithful to the original, with changes to presentation, spelling, and grammar as needed. The PMCID and DOI were added when they were missing from the original reference. No citation was given for RIQAS in the original; a citation was provided for this version. No other changes were made in accordance with the "NoDerivatives" portion of the content license.