Journal:Digitalization of calibration data management in the pharmaceutical industry using a multitenant platform
| Full article title | Digitalization of calibration data management in the pharmaceutical industry using a multitenant platform |
|---|---|
| Journal | Applied Sciences |
| Author(s) | Mustapää, Tuukka; Nummiluikki, Juho; Viitala, Raine |
| Author affiliation(s) | Aalto University, Beamex Oy Ab |
| Primary contact | Email: tuukka dot mustapaa at aalto dot fi |
| Editors | Mendyk, Aleksander |
| Year published | 2022 |
| Volume and issue | 12(15) |
| Article # | 7531 |
| DOI | 10.3390/app12157531 |
| ISSN | 2076-3417 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.mdpi.com/2076-3417/12/15/7531 |
| Download | https://www.mdpi.com/2076-3417/12/15/7531/pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
The global quality infrastructure (QI) has been established and is maintained to ensure the safety of products and services for their users. One of the cornerstones of the QI is metrology, i.e., the science of measurement, as quality management systems (QMS) commonly rely on measurements for evaluating quality. For this reason, calibration procedures and management of the data related to them are of the utmost importance for quality management in the process industry, made a particularly high priority by regulatory authorities. To overcome the relatively low level of digitalization in metrology, machine-interpretable data formats such as digital calibration certificates (DCC) are being developed.
In this paper, we analyze the current calibration processes in the pharmaceutical industry, and the requirements defined for them in the relevant standards and regulations. For digitalizing the calibration-related data exchange, a multitenant cloud- and platform-based method is presented. To test and validate the approach, a proof of concept (POC) implementation of the platform is developed with a focus on ease and cost-efficiency of deployment and use, while also ensuring the preservation of traceability and data integrity. The POC is based on two industrial use cases involving organizations with different roles in the metrology infrastructure. From testing this POC, the presented approach proves to be an efficient method for organizing the calibration data exchange in industrial use.
Keywords: digitalization, quality management, pharmaceutical industry, digital calibration certificate, metrology, traceability, platform
Introduction
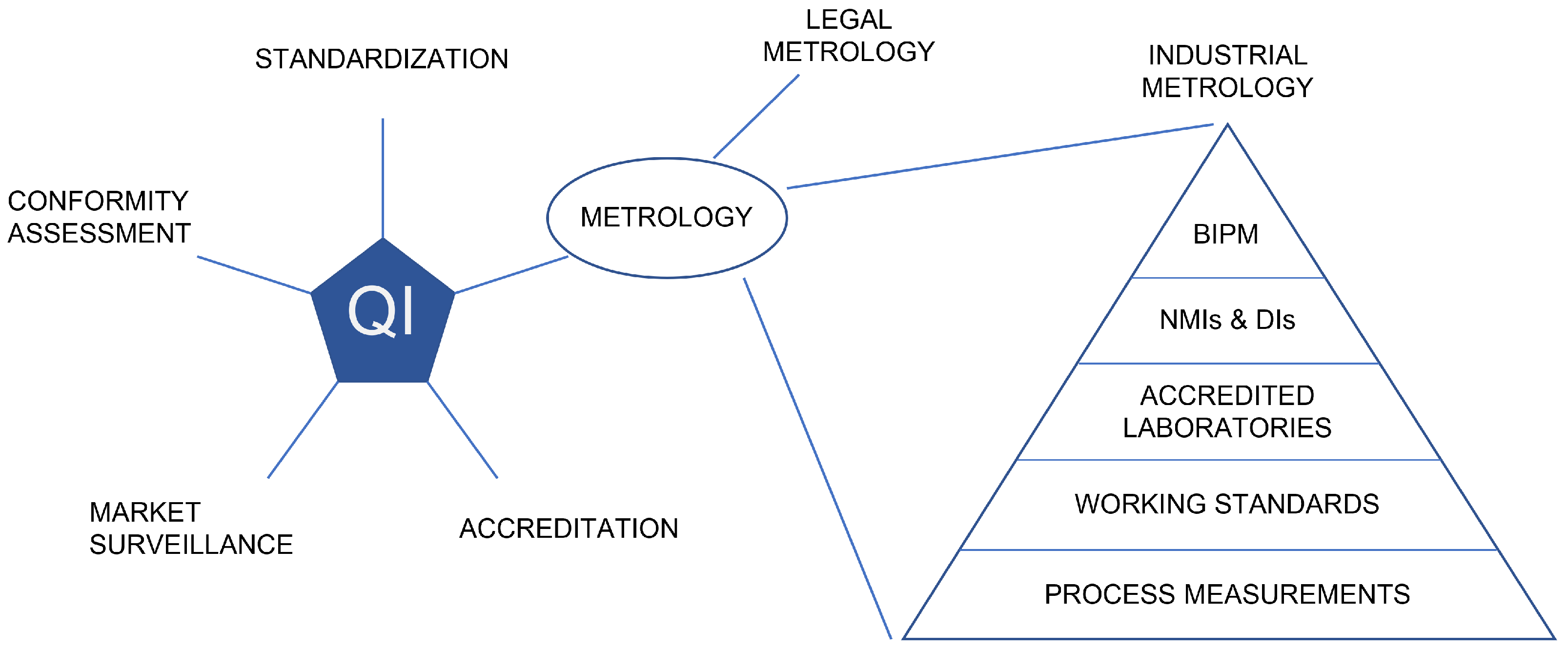
Whenever a product or service is brought to market, it is crucial that the product or service in question is safe for consumers and the environment. For these purposes, a global quality infrastructure (QI) consisting of both public and private organizations has been established. [1] The global cooperation of the QI is coordinated by the International Network on Quality Infrastructure (INetQI) [3], which defines QI as "the system comprising the organizations (public and private) together with the policies, relevant legal and regulatory framework, and practices needed to support and enhance the quality, safety and environmental soundness of goods, services and processes." [1] The cornerstones of QI are metrology, standardization, accreditation, conformity assessment, and market surveillance, which have their own sub-infrastructures, such as metrology infrastructure (MI), which is tied to its own international organizations. [1,2] Due to the differences in roles of the organizations and regulatory frameworks in the QI, there are organizations and regulatory bodies covering the different parts of the QI on national or regional levels. In turn, due to this division, there are possibilities for overlapping or conflicting regulations, which regulatory bodies ideally aim to avoid.
Currently, as significant efforts are being made to digitalize the QI [1,4,5,6,7], the differences in current practices and requirements quickly become apparent, causing challenges in the implementation of digital solutions. For ensuring that the data formats and digital processes meet their respective requirements and are applicable globally in different applications, harmonization is needed, as the current requirements may vary by the domain or region. Otherwise, it could be impossible, or at least highly cost-inefficient, for the organizations participating in the maintenance of the infrastructures, such as calibration laboratories and service providers, to adapt their services to be interoperable with the varying systems used by individual customers.
In this paper, we focus on the industrial part of the MI, in which the determining of the measurement uncertainty of individual instruments and traceability of measurements to the measurement unit system are established through calibrations. [8] As calibrations are a confirmative part of the quality management of processes that are based on measurements, they are not directly profitable operations but instead are necessary for avoiding prohibitive expenses and delays or fulfilling compliance requirements, allowing access to markets. For this reason, estimating the total economic benefits of the digital transformation of the MI for all the involved organizations individually is complex.
The same also applies to other similar processes in different parts of the QI. As the purpose of the QI, and thus also the MI, is mainly to oversee and support the industry in providing reliable and safe products and services to customers [1], most economical benefits from the digital transformation are formed in the customer end of the infrastructure. In the MI, this includes, e.g., manufacturing companies that can have several thousands of measurement instruments monitoring and controlling production lines and processes. For these companies, a significant part of the value of digitalization comes from the improvements in efficiency and reduced need for manual work through automation, which leads to savings from reduced human resources tied to quality management. In the case of calibrations, where the costs and possible savings from managing instrument data and information are relative to the number of the instruments that need to be regularly calibrated, these benefits will not be as significant for smaller service providers. This means that any investments in the digital transformation become difficult to justify without support and demand from the customers.
A good example of an industry that is reliant on MI is the pharmaceutical industry, where measurements provide the means for controlling the drug manufacturing processes. Thus, the quality and safety of pharmaceutical products are directly dependent on the reliability of process measurements. For this reason, ensuring the trustworthiness of the process instruments is an essential part of quality management in the pharmaceutical industry, which is why the measurement systems and their maintenance and calibration procedures are highly regulated. [9,10,11] Ensuring the data integrity of the measurements and any calibration-related data in particular is of the utmost importance for this purpose, which is why detailed records and audit trails for the processes are required. [12] Consequently, any processes including human operations, e.g., related to the documentation and handling of calibration data, will typically require several inspection and approval procedures to prevent the occurrence of human errors.
Since harmonization of data formats is essential for the interoperability and overall efficiency of digital systems, important areas requiring in-depth examination are the established standards, regulations, and guidelines that provide the framework for the current data formats and processes. Thus, a key question for the success of digitalization efforts becomes, "how compatible and applicable are the requirements defined for the different parties in QI in a fully digital environment?"
In general, the success of industry-wide transition in the digital environment relies on organizational capabilities to adapt to and uptake new technologies and systems when necessary. Thus, an important aspect of the digital transformation is ensuring the ease of adaptation, inexpensiveness, and sufficient scalability of the processes and systems so that the requirements of various types and sizes of organizations can be fulfilled. One possible solution for arranging these kinds of systems and services in a scalable manner is the utilization of a common cloud platform. An example of such a concept in the domain of metrology is the European Metrology Cloud project, aiming to advance the digitalization of legal metrology in Europe. [7,13]
Optimal digitalization of calibration data management requires a thorough understanding of the underlying processes and workflows. In this paper, we investigate the current practices and general requirements for calibration data management as a part of an overall quality management system (QMS) in the pharmaceutical industry. We analyze the significance and feasibility of the requirements for the harmonization of digital processes. The paper presents ways to optimize calibration data management processes, allowing improved efficiency by reduced manual work and removing the possibility of human errors in calibration data management. Thus, traceability and data integrity can be preserved between the organizations in the calibration chain. Furthermore, the proposed approach enables data analysis of the calibration data on a completely different scale compared to the printed A4/paper on glass (e.g., PDF) approach. A proof of concept (POC) of the method was developed by prioritizing the ease of adaptation to digital processes, and as such, the POC used systems already widely used in the industry, e.g., for user and access management. Thus, the set-up and operation of the platform required a less complex infrastructure compared to a fully proprietary infrastructure, as used by Thiel [13] and Bruns et al. [14]
To summarize, we present the following contributions beyond the state-of-the-art:
- Investigation of calibration procedures in the context of digital calibration certificates (DCC) [15], including the core standards and regulations affecting the pharmaceutical industry;
- Analysis of the applicability of the standards and regulations for the implementation of machine-proof data formats and application programming interfaces (APIs), acknowledging that the existing procedures, standards, and regulations have been prepared for human interpretation;
- Optimized digital data management processes for preserving traceability and data integrity in calibration chains;
- A concept for a multitenant platform for establishing ecosystems for collaborating organizations within the metrology infrastructure; and
- A proof of concept (POC) realization of points 3 and 4.
he paper is organized as follows. The next section provides the relevant background on the digitalization of metrology infrastructure, current developments in the use of internet of things (IoT) in manufacturing and quality management, and requirements for calibration data management in the process and pharmaceutical industries. Next, the harmonization of digitalized calibration data management based on the requirements for different organizations is discussed, followed by the proposed approach for the exchange of digital calibration data within the metrology infrastructure. The possibilities of the digital calibration data management, remaining challenges, and proposed research topics are discussed in the penultimate section, followed by conclusions.
Background
Metrology infrastructure as a part of the quality infrastructure
The worldwide MI is built upon standards, mutual trust, and recognition among organizations operating around the world. [16] To make the infrastructure as comprehensive as possible, it consists of organizations with different hierarchical roles. At the top of the hierarchy are the national metrology institutes (NMIs) and designated institutes (DI), which maintain the SI system and metrological standards jointly defined by the International Bureau of Weights and Measures (BIPM). Figure 1 illustrates how the MI is established as a part of the QI. The hierarchy of the MI is often depicted in the form of a triangle or pyramid as the amount of measurement instruments and references increases when moving from the NMIs towards the industrial measurement application. (A few examples of how the MI has been implemented nationally in European countries are given in the work of Nikander et al.[17].)
|
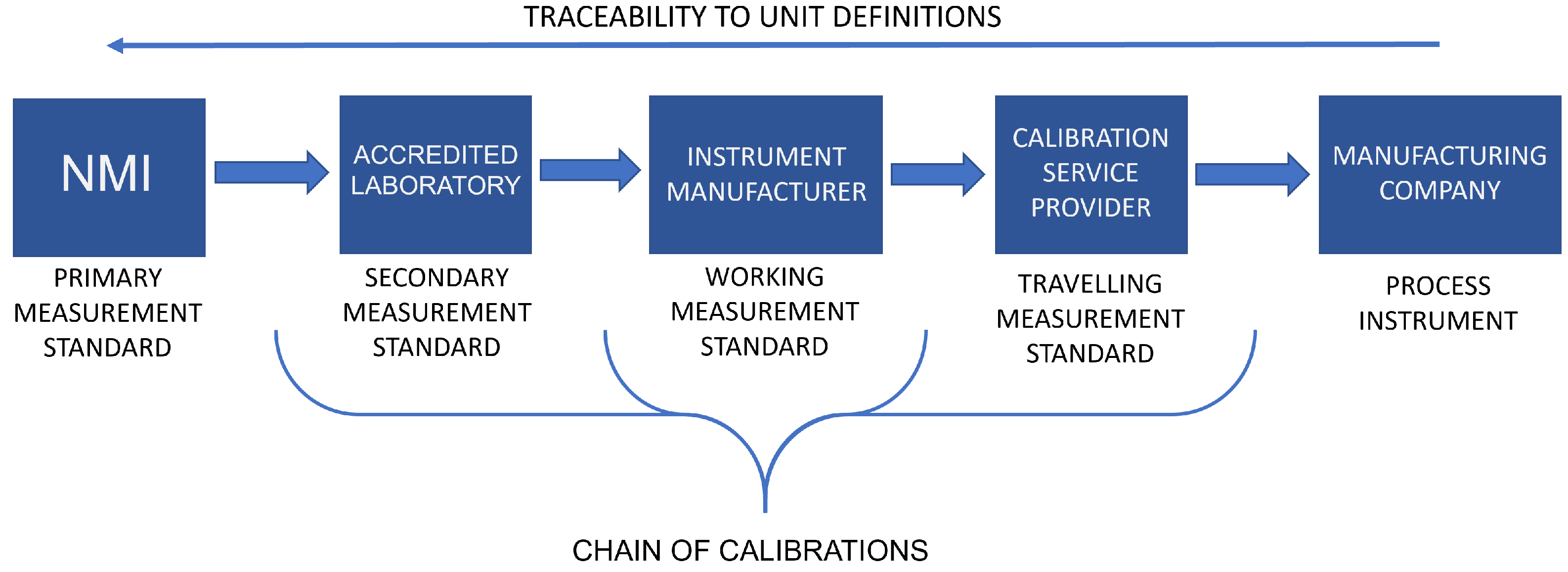
The measurement standards maintained by the NMIs or DIs are referred to as primary standards, as their purpose is to provide as accurate and precise physical representations of the unit definitions as possible. Between the industrial applications and primary standards are secondary measurement standards, which are maintained by accredited laboratories and calibrated by the NMIs or DIs. Calibrations are essential to maintain the traceability to SI unit definitions, which ensures the comparability and trustworthiness of measurement results given by individual instruments. [8] Depending on the applications, additional levels of references may also be used in the industry. For example, in the pharmaceutical industry, where the number of instruments used for monitoring and controlling the manufacturing processes can be several thousand per manufacturing site, the use of working standards and travelling standards is common as they provide efficiency in terms of time and costs, e.g., by allowing the calibrations of the process instruments to be carried out on-site. Figure 2 shows an example of a calibration chain from the primary standard of an NMI, all the way through to a process instrument of a pharmaceutical manufacturing company.
|
Due to the complexity of the MI and diversity of the organizations involved, the progress of digitalization has been slow, and in many cases data are still being managed in paper or paper-on-glass formats, requiring human interpretation, e.g., in the case of documenting calibrations in calibration certificates. [15] For field calibrations, digitalized solutions have become more common, as the costs of calibrations are proportional to the efficiency of the processes. However, these solutions tend to be instrument- and system-provider-specific, and due to competition, the willingness of the system providers to collaborate by harmonizing their systems and making them more open has been low. In addition, it is common that larger industrial companies cooperate with several instrument manufacturers and service providers. Consequently, in the worst case for the instrument owner, the data exchange from different organizations has been scattered in several systems using different formats. Because of this, human resources relative to the number of annual calibrations have been tied up in calibration management alone to ensure compliance with quality management regulations, meaning that it has been a significant expense.
As digitalization in the industry and other sectors is progressing, and the collection and use of data is growing rapidly, the topics concerning data quality and trustworthiness become increasingly important, which is driving the research and development efforts in digitalization within the metrology community. [7,18] The ongoing efforts include the definition and development of digital formats for presenting metrological information, e.g., data models for presenting metrologically relevant data as semantic metadata [19,20,21], or formats for digital calibration certificates. [15,22,23,24] For this work, the exchange of DCCs was tested using the DCC format originally defined by the Physikalisch-Technische Bundesanstalt (PTB) and further developed in EMPIR SmartCom and Gemimeg research projects. [4,5,25,26,27,28]
Digitization of the data formats sets new requirements for the development of the infrastructure, as its operation has been based on mutual recognition and trust between organizations. [16] Transition to a digital environment also means that this trust needs to be established digitally to enable its representation in a machine-understandable format. [29,30,31] For this purpose, data security and cryptographical solutions, e.g., the use of digital signatures, are relevant. [15,17]
IoT in pharmaceutical and process industries
The digital transformation of the manufacturing industry is typically referred to as Industry 4.0, based on the significance and potential of digitalization in industrial settings. The key technologies of Industry 4.0 include smart IoT devices and sensors that enable the collection of vast amounts of data from different manufacturing processes and operations. Combined with the technological advancements in computing power, analyzing methods for big data and machine learning, Industry 4.0 enables significant improvements in the efficiency of manufacturing processes. [32]
In addition to automation, digitalization also provides new possibilities to enhance the work where automation is not feasible or needs to be supported by manual operations, i.e., smart working. Smart-working-enabling technologies include, e.g., wearable IoT devices and augmented reality, which aid the interaction between the operators and manufacturing systems. [33] IoT technologies can also be used to improve worker safety in the working environment. [34]
One of the main technologies that is being studied and developed based on Industry-4.0-enabling technologies is cyber-physical systems, i.e., digital twins, in which, e.g., data, simulation models, and predictive analytics are used to form as accurate a digital representation of a physical entity as possible to further improve the possibilities to analyze their performance and behavior. [35,36,37,38]
In terms of quality management, the effects of Industry 4.0 are prominent in the digitalization of methodologies such as total quality management (TQM) and the use of IoT technologies in quality management operations and processes. [39] In some cases, the advancements in IoT technologies have also led to situations where traditional quality management is not in alignment with the requirements of IoT devices. [40]
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Grammar was tweaked significantly to improve readability. The PMCID and DOI were also added when they were missing from the original reference.