Difference between revisions of "Journal:Error evaluation in the laboratory testing process and laboratory information systems"
Shawndouglas (talk | contribs) m (OMBOX) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 38: | Line 38: | ||
==Introduction== | ==Introduction== | ||
A mistake or inefficiency in one of the stages of the [[laboratory]] testing chain can affect overall process implementation and management, and subsequently physician diagnosis. (1, 2) However, a [[laboratory information system]] (LIS) is able to expedite and facilitate these and other interactions during the laboratory testing process. (3) Yet involvement of multiple units in testing [[workflow]] still requires effective use of the LIS to monitor task performance, ensure a smooth process, and readily identify errors. A complex, error prone, unreliable, and poorly designed LIS, on the other hand, may introduce errors identifiable in laboratory test results. (4, 5) These outcomes can be further aggravated when the LIS links erroneous patient and test data to other units and institutions and involves data exchange because of complex intersystem interaction. (6) Further errors can also be introduced, attributable to human factors, including patient misidentification and erroneous test requests. (7) | |||
To minimizes these errors, some labs have turned to the total testing process (TTP) (8), a unique framework that guides the testing process, and analyzes and minimizes testing error risk not only in the core laboratory but also in other clinical units.(7, 9) The TTP includes internal and external laboratory activities that involve one or more procedures requiring staff interaction. However, the TTP should also address the role the LIS plays in the testing process. | |||
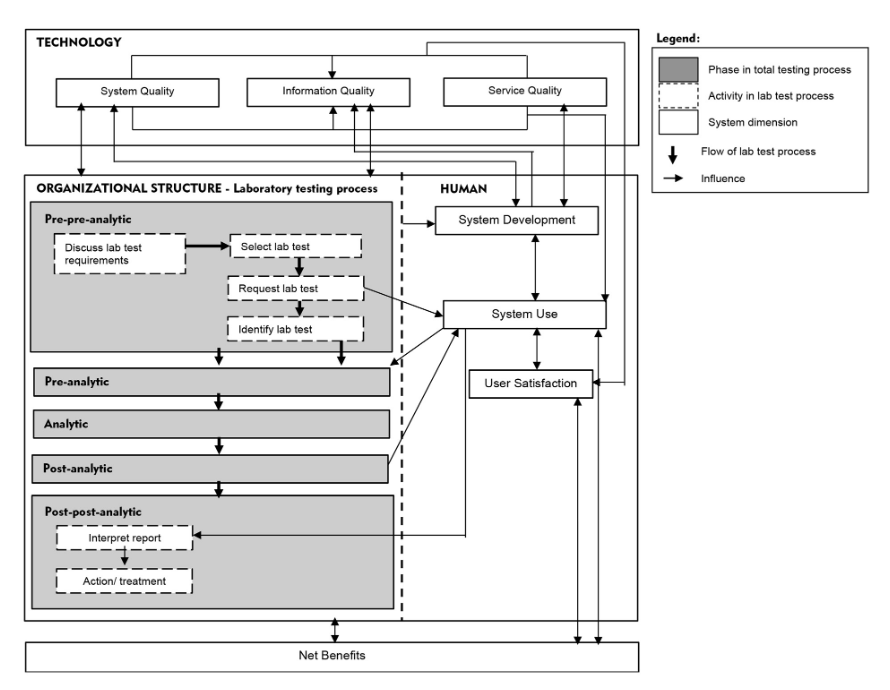
We propose a "total testing process for laboratory information systems" (TTP-LIS) framework on the basis of a combination of TTP and human, organization, technology, and fit (HOT-fit) frameworks. (10, 11) The HOT aspects are crucial elements that complement the evaluation of the LIS and lab testing process. The proposed framework aims to illustrate a systematic, coordinated, and optimized laboratory testing process and LIS workflow to facilitate a rigorous error evaluation process. (12) The evaluation factors, dimensions, measures, and their relationships are depicted in Figure 1. | |||
[[File:Fig1 Arifin JofMedBio21 40.png|890px]] | |||
{{clear}} | |||
{| | |||
| STYLE="vertical-align:top;"| | |||
{| border="0" cellpadding="5" cellspacing="0" width="890px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Figure 1.''' The proposed TTP-LIS framework</blockquote> | |||
|- | |||
|} | |||
|} | |||
==Abbreviations== | ==Abbreviations== | ||
Revision as of 18:44, 27 October 2021
| Full article title | Error evaluation in the laboratory testing process and laboratory information systems |
|---|---|
| Journal | Journal of Medical Biochemistry |
| Author(s) | Arifin, Azila; Yusof, Maryati |
| Author affiliation(s) | Universiti Kebangsaan Malaysia |
| Primary contact | Email: Maryati dot Yusof at ukm dot edu dot my |
| Year published | 2021 |
| Volume and issue | 40 |
| Page(s) | 1–11 |
| DOI | 10.5937/jomb0-31382 |
| ISSN | 1452-8258 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://aseestant.ceon.rs/index.php/jomb/article/view/31382 |
| Download | https://aseestant.ceon.rs/index.php/jomb/article/view/31382/17766 (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Background: The laboratory testing process consists of five analysis phases, featuring the total testing process (TTP) framework. Activities in laboratory processing, including those of testing, are error-prone and affect the use of laboratory information systems (LIS). This study seeks to identify error factors related to system use, as well as the first and last phases of the laboratory testing process, using a proposed framework known as the "total testing process for laboratory information systems" (TTP-LIS).
Methods: We conducted a qualitative case study evaluation in two private hospitals and a medical laboratory. We collected data using interviews, observations, and document analysis methods involving physicians, nurses, an information technology officer, and the laboratory staff. We employed the proposed framework and lean problem solving tools, namely value-stream mapping and the A3 process for data analysis.
Results: Errors in an LIS and the laboratory testing process were attributed to failure to fulfill user requirements, poor cooperation between the information technology unit and laboratory, inconsistency of software design in system integration, errors during inter-system data transmission, and lack of motivation in system use. The error factors are related to system development elements, namely latent failures that considerably affected information quality and system use. Errors in system development were also attributed to poor service quality.
Conclusions: Complex laboratory testing processes and LISs require rigorous evaluation in minimizing errors and ensuring patient safety. The proposed framework and lean approach are applicable for evaluating the laboratory testing process and LISs in a rigorous, comprehensive, and structured manner.
Keywords: case study, error, evaluation, framework, laboratory information system, lean, patient safety, total testing process, socio-technical
Introduction
A mistake or inefficiency in one of the stages of the laboratory testing chain can affect overall process implementation and management, and subsequently physician diagnosis. (1, 2) However, a laboratory information system (LIS) is able to expedite and facilitate these and other interactions during the laboratory testing process. (3) Yet involvement of multiple units in testing workflow still requires effective use of the LIS to monitor task performance, ensure a smooth process, and readily identify errors. A complex, error prone, unreliable, and poorly designed LIS, on the other hand, may introduce errors identifiable in laboratory test results. (4, 5) These outcomes can be further aggravated when the LIS links erroneous patient and test data to other units and institutions and involves data exchange because of complex intersystem interaction. (6) Further errors can also be introduced, attributable to human factors, including patient misidentification and erroneous test requests. (7)
To minimizes these errors, some labs have turned to the total testing process (TTP) (8), a unique framework that guides the testing process, and analyzes and minimizes testing error risk not only in the core laboratory but also in other clinical units.(7, 9) The TTP includes internal and external laboratory activities that involve one or more procedures requiring staff interaction. However, the TTP should also address the role the LIS plays in the testing process.
We propose a "total testing process for laboratory information systems" (TTP-LIS) framework on the basis of a combination of TTP and human, organization, technology, and fit (HOT-fit) frameworks. (10, 11) The HOT aspects are crucial elements that complement the evaluation of the LIS and lab testing process. The proposed framework aims to illustrate a systematic, coordinated, and optimized laboratory testing process and LIS workflow to facilitate a rigorous error evaluation process. (12) The evaluation factors, dimensions, measures, and their relationships are depicted in Figure 1.
|
Abbreviations
LIS: laboratory information system TTP: total testing process TTP-LIS: total testing process for laboratory information system
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation, spelling, and grammar. In some cases important information was missing from the references, and that information was added.