Journal:Error evaluation in the laboratory testing process and laboratory information systems
| Full article title | Error evaluation in the laboratory testing process and laboratory information systems |
|---|---|
| Journal | Journal of Medical Biochemistry |
| Author(s) | Arifin, Azila; Yusof, Maryati |
| Author affiliation(s) | Universiti Kebangsaan Malaysia |
| Primary contact | Email: Maryati dot Yusof at ukm dot edu dot my |
| Year published | 2021 |
| Volume and issue | 40 |
| Page(s) | 1–11 |
| DOI | 10.5937/jomb0-31382 |
| ISSN | 1452-8258 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://aseestant.ceon.rs/index.php/jomb/article/view/31382 |
| Download | https://aseestant.ceon.rs/index.php/jomb/article/view/31382/17766 (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Background: The laboratory testing process consists of five analysis phases, featuring the total testing process (TTP) framework. Activities in laboratory processing, including those of testing, are error-prone and affect the use of laboratory information systems (LIS). This study seeks to identify error factors related to system use, as well as the first and last phases of the laboratory testing process, using a proposed framework known as the "total testing process for laboratory information systems" (TTP-LIS).
Methods: We conducted a qualitative case study evaluation in two private hospitals and a medical laboratory. We collected data using interviews, observations, and document analysis methods involving physicians, nurses, an information technology officer, and the laboratory staff. We employed the proposed framework and lean problem solving tools, namely value-stream mapping and the A3 process for data analysis.
Results: Errors in an LIS and the laboratory testing process were attributed to failure to fulfill user requirements, poor cooperation between the information technology unit and laboratory, inconsistency of software design in system integration, errors during inter-system data transmission, and lack of motivation in system use. The error factors are related to system development elements, namely latent failures that considerably affected information quality and system use. Errors in system development were also attributed to poor service quality.
Conclusions: Complex laboratory testing processes and LISs require rigorous evaluation in minimizing errors and ensuring patient safety. The proposed framework and lean approach are applicable for evaluating the laboratory testing process and LISs in a rigorous, comprehensive, and structured manner.
Keywords: case study, error, evaluation, framework, laboratory information system, lean, patient safety, total testing process, socio-technical
Introduction
A mistake or inefficiency in one of the stages of the laboratory testing chain can affect overall process implementation and management, and subsequently physician diagnosis.[1][2] However, a laboratory information system (LIS) is able to expedite and facilitate these and other interactions during the laboratory testing process.[3] Yet involvement of multiple units in testing workflow still requires effective use of the LIS to monitor task performance, ensure a smooth process, and readily identify errors. A complex, error prone, unreliable, and poorly designed LIS, on the other hand, may introduce errors identifiable in laboratory test results.[4][5] These outcomes can be further aggravated when the LIS links erroneous patient and test data to other units and institutions and involves data exchange because of complex intersystem interaction.[6] Further errors can also be introduced, attributable to human factors, including patient misidentification and erroneous test requests.[7]
To minimizes these errors, some labs have turned to the total testing process (TTP)[8], a unique framework that guides the testing process, and analyzes and minimizes testing error risk not only in the core laboratory but also in other clinical units.[7][9] The TTP includes internal and external laboratory activities that involve one or more procedures requiring staff interaction. However, the TTP should also address the role the LIS plays in the testing process.
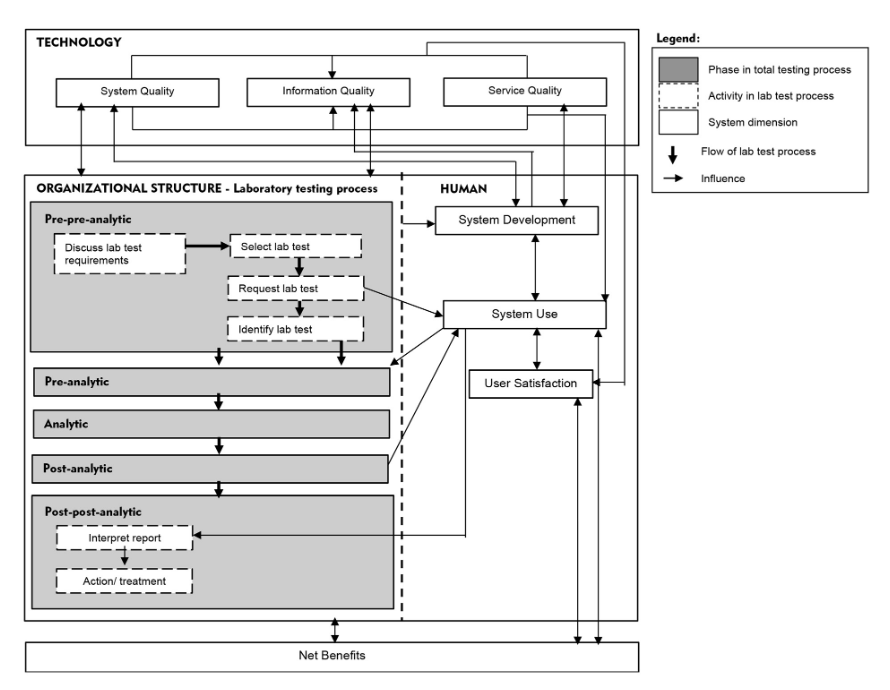
We propose a "total testing process for laboratory information systems" (TTP-LIS) framework on the basis of a combination of TTP and human, organization, technology, and fit (HOT-fit) frameworks.[10][11] The HOT aspects are crucial elements that complement the evaluation of the LIS and lab testing process. The proposed framework aims to illustrate a systematic, coordinated, and optimized laboratory testing process and LIS workflow to facilitate a rigorous error evaluation process.[12] The evaluation factors, dimensions, measures, and their relationships are depicted in Figure 1.
|
Error evaluation can benefit from lean, a quality improvement method that emphasizes removing process waste, including error. Various lean tools—such as value-stream mapping (VSM), 5 Why, and A3 problem-solving methods—have been widely used for process improvement.[13] A3 takes a structured approach to problem solving that uses a report tool to summarize the definition, scope, discovery process, findings, proposed action steps, and results from the problem analysis. A3 can be combined with other lean tools, such as VSM and 5 Why, to visualize and identify the root cause of problems. VSM is used to illustrate the overall process to identify waste and other problems and the appropriate solutions in the current and future state map, respectively. The problem can be scrutinized using the 5 Why tool to identify its root cause and mitigation strategy by asking a series of questions, either five times or any appropriate range. This study focused only on pre-pre-analytic and post-post-analytic phases of the TTP framework, given their high error rates[14][15], compared to other phases.
Material and methods
We conducted a subjectivist case study strategy employing qualitative methods in this summative evaluation to examine errors related to the LIS and the first and last phases of the lab testing process. A subjectivist approach enabled a comprehensive understanding of the healthcare context surrounding the management of LIS-induced error by generating detailed, insightful explanations.[16][17] We performed the evaluation by applying the TTP-LIS framework at two premier private hospitals in Malaysia. These cutting-edge hospitals have lead national health care efforts and are recognized by accreditation bodies such as the Malaysian Society for Quality in Health, Joint Commission International (XI), and Quality Management System (MS ISO 9001: 2015). The local Institutional Review Board deemed this study exempt from review. Author AA, a trained qualitative researcher, collected the data through interviews, non-participant observations, and document/artifact analysis methods.
Sampling
A purposeful snowball sampling method provided in-depth information from key informants. We identified participants from our initial contact with the lab director. We discussed the appropriateness of selected informants with the lab head based on the irrespective expertise, job scope, and abilities in providing the required information. Finally, we recruited 15 participants, including clinicians and management, lab, and IT staff (Table 1).
| ||||||||||||||||||
Data collection and analysis methods
The face-to-face, one-on-one interviews lasted for one to two hours for each informant who we queried on lab testing processes, LIS use, error and mistake incidents, their causes, and the strategies for mitigation and LIS improvement. We recorded (audio) and transcribed interviews. Observation took place in a medical lab for over a day on lab testing processes, from clinical requests to the production of lab results, to identify potential LIS-induced errors. We analyzed documents related to the LIS’ overall development, operation and management, process owner, backup system handling, and software and hardware management. We analyzed data thematically using the initial TTP-LIS evaluation framework.[12] In addition, we employed three lean tools—VSM, 5 Why, and A3—to visualize the current process, its problems, and root causes, as well as the desired (future) state of the first and last phases of lab testing.[13] We validated and refined the TTP framework with an expert who reviewed and acknowledged the said framework as a comprehensive evaluation tool for the lab testing process and LIS usage.
Abbreviations
HOT-fit: human, organization, technology, and fit framework
LIS: laboratory information system
TTP: total testing process
TTP-LIS: total testing process for laboratory information system
VSM : value-stream mapping
References
- ↑ Dighe, AS; Baron, JM (2011). "Computerized provider order entry in the clinical laboratory" (in en). Journal of Pathology Informatics 2 (1): 35. doi:10.4103/2153-3539.83740. ISSN 2153-3539. PMC PMC3162747. PMID 21886891. http://www.jpathinformatics.org/text.asp?2011/2/1/35/83740.
- ↑ Carraro, Paolo; Zago, Tatiana; Plebani, Mario (1 March 2012). "Exploring the Initial Steps of the Testing Process: Frequency and Nature of Pre-Preanalytic Errors" (in en). Clinical Chemistry 58 (3): 638–642. doi:10.1373/clinchem.2011.175711. ISSN 0009-9147. https://academic.oup.com/clinchem/article/58/3/638/5620542.
- ↑ Yeo, Cp; Ng, Wy (1 November 2018). "Automation and productivity in the clinical laboratory: experience of a tertiary healthcare facility". Singapore Medical Journal: 597–601. doi:10.11622/smedj.2018136. PMC PMC6250760. PMID 30498842. http://www.smj.org.sg/article/automation-and-productivity-clinical-laboratory-experience-tertiary-healthcare-facility.
- ↑ Bowie, Paul; Price, Julie; Hepworth, Neil; Dinwoodie, Mark; McKay, John (1 November 2015). "System hazards in managing laboratory test requests and results in primary care: medical protection database analysis and conceptual model" (in en). BMJ Open 5 (11): e008968. doi:10.1136/bmjopen-2015-008968. ISSN 2044-6055. PMC PMC4663465. PMID 26614621. https://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2015-008968.
- ↑ Mathews, Althea; Marc, David (2017). "Usability evaluation of laboratory information systems" (in en). Journal of Pathology Informatics 8: 40. doi:10.4103/jpi.jpi_24_17. ISSN 2153-3539. PMC PMC5653961. PMID 29114434. http://www.jpathinformatics.org/text.asp?2017/8/1/40/215895.
- ↑ Rajamani, Sripriya; Kayser, Ann; Emerson, Emily; Solarz, Sarah (21 September 2018). "Evaluation of Data Exchange Process for Interoperability and Impact on Electronic Laboratory Reporting Quality to a State Public Health Agency". Online Journal of Public Health Informatics 10 (2): e204. doi:10.5210/ojphi.v10i2.9317. ISSN 1947-2579. PMC PMC6194099. PMID 30349622. https://journals.uic.edu/ojs/index.php/ojphi/article/view/9317.
- ↑ 7.0 7.1 Sonmez, Cigdem; Yıldız, Ummugulsum; Akkaya, Nedim; Taneli, Fatma (20 March 2020). "Preanalytical Phase Errors: Experience of a Central Laboratory" (in en). Cureus 12 (3): e7335. doi:10.7759/cureus.7335. ISSN 2168-8184. PMC PMC7164707. PMID 32313776. https://www.cureus.com/articles/28897-preanalytical-phase-errors-experience-of-a-central-laboratory.
- ↑ Lundberg, G.D (1 February 1999). "How clinicians should use the diagnostic laboratory in a changing medical world" (in en). Clinica Chimica Acta 280 (1-2): 3–11. doi:10.1016/S0009-8981(98)00193-4. https://linkinghub.elsevier.com/retrieve/pii/S0009898198001934.
- ↑ Plebani, Mario; Piva, Elisa (1 October 2010). "Medical Errors: Pre-Analytical Issue in Patient Safety". Journal of Medical Biochemistry 29 (4): 310–314. doi:10.2478/v10011-010-0039-2. ISSN 1452-8266. https://scindeks.ceon.rs/article.aspx?artid=1452-82581004310P.
- ↑ Yusof, Maryati Mohd. (1 July 2015). "A case study evaluation of a Critical Care Information System adoption using the socio-technical and fit approach" (in en). International Journal of Medical Informatics 84 (7): 486–499. doi:10.1016/j.ijmedinf.2015.03.001. https://linkinghub.elsevier.com/retrieve/pii/S1386505615000611.
- ↑ Yusof, Maryati Mohd.; Kuljis, Jasna; Papazafeiropoulou, Anastasia; Stergioulas, Lampros K. (1 June 2008). "An evaluation framework for Health Information Systems: human, organization and technology-fit factors (HOT-fit)" (in en). International Journal of Medical Informatics 77 (6): 386–398. doi:10.1016/j.ijmedinf.2007.08.011. https://linkinghub.elsevier.com/retrieve/pii/S1386505607001608.
- ↑ 12.0 12.1 Carraro, Paolo; Plebani, Mario (1 July 2007). "Errors in a Stat Laboratory: Types and Frequencies 10 Years Later" (in en). Clinical Chemistry 53 (7): 1338–1342. doi:10.1373/clinchem.2007.088344. ISSN 0009-9147. https://academic.oup.com/clinchem/article/53/7/1338/5627526.
- ↑ 13.0 13.1 Lippi, Giuseppe; Betsou, Fay; Cadamuro, Janne; Cornes, Michael; Fleischhacker, Michael; Fruekilde, Palle; Neumaier, Michael; Nybo, Mads et al. (26 June 2019). "Preanalytical challenges – time for solutions" (in en). Clinical Chemistry and Laboratory Medicine (CCLM) 57 (7): 974–981. doi:10.1515/cclm-2018-1334. ISSN 1437-4331. https://www.degruyter.com/document/doi/10.1515/cclm-2018-1334/html.
- ↑ Friedman, Charles P.; Wyatt, J. (2006). Evaluation methods in biomedical informatics. Health informatics (2nd ed ed.). New York: Springer. ISBN 978-0-387-25889-8.
- ↑ Jimmerson, Cindy LeDuc (2010). Value stream mapping for healthcare made easy. Boca Raton: CRC Press. ISBN 978-1-4200-7852-7. OCLC 301879954. https://www.worldcat.org/title/mediawiki/oclc/301879954.
- ↑ Pitt, Leyland F.; Watson, Richard T.; Kavan, C. Bruce (1 June 1995). "Service Quality: A Measure of Information Systems Effectiveness". MIS Quarterly 19 (2): 173-187. doi:10.2307/249687. https://www.jstor.org/stable/249687?origin=crossref.
- ↑ Croxatto, A.; Prod'hom, G.; Faverjon, F.; Rochais, Y.; Greub, G. (1 March 2016). "Laboratory automation in clinical bacteriology: what system to choose?" (in en). Clinical Microbiology and Infection 22 (3): 217–235. doi:10.1016/j.cmi.2015.09.030. https://linkinghub.elsevier.com/retrieve/pii/S1198743X16000069.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation, spelling, and grammar. In some cases important information was missing from the references, and that information was added.