Difference between revisions of "Journal:Guideline for software life cycle in health informatics"
Shawndouglas (talk | contribs) (Created stub. Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 20: | Line 20: | ||
}} | }} | ||
==Abstract== | ==Abstract== | ||
The long-lasting trend of medical informatics is to adapt novel technologies in the medical context. In particular, incorporating artificial intelligence to support clinical decision-making can significantly improve monitoring, diagnostics, and prognostics for the patient’s and medic’s sake. However, obstacles hinder a timely technology transfer from research to the clinic. Due to the pressure for novelty in the research context, projects rarely implement quality standards. | The long-lasting trend of [[medical informatics]] is to adapt novel technologies in the medical context. In particular, incorporating [[artificial intelligence]] (AI) to support clinical decision-making can significantly improve monitoring, diagnostics, and prognostics for the patient’s and medic’s sake. However, obstacles hinder a timely technology transfer from research to the clinic. Due to the pressure for novelty in the [[research]] context, projects rarely implement [[Quality (business)|quality]] standards. | ||
Here, we propose a guideline for academic software life cycle processes tailored to the needs and capabilities of research organizations. While the complete implementation of a software life cycle according to commercial standards is not feasible in scientific work, we propose a subset of elements that we are convinced will provide a significant benefit while keeping the effort within a feasible range. | Here, we propose a guideline for academic software life cycle processes tailored to the needs and capabilities of research organizations. While the complete implementation of a software life cycle according to commercial standards is not feasible in scientific work, we propose a subset of elements that we are convinced will provide a significant benefit while keeping the effort within a feasible range. | ||
| Line 28: | Line 28: | ||
'''Keywords''': health informatics, bioinformatics, software engineering | '''Keywords''': health informatics, bioinformatics, software engineering | ||
'''Graphical abstract''': [[File:GA | '''Graphical abstract''': [[File:GA Hauschild iScience2022 25-12.jpg|400px]] | ||
{{clear}} | {{clear}} | ||
==Introduction== | |||
Today, [[medical informatics]] is an integral part of health care systems that ensure the smooth operation of processes in medical care. Moreover, standard procedures ensure the transfer of knowledge from medical [[research]] to clinical practice, for instance, via regularly updated guidelines and [[Regulatory compliance|regulations]]. In contrast, newly developed software innovations—such as systems based on [[artificial intelligence]] (AI) that could support clinical decisions and have already evolved to be the state-of-the-art in medical informatics research—rarely transfer to application in practice. | |||
Modern methods such as AI and [[machine learning]] (ML) increasingly unroll their potential in medical healthcare to help patients and clinicians. [1] [[Clinical decision support system]]s (CDSSs) can effectively increase diagnostics, patient safety, and cost containment. [2] Easily accessible AI-based applications can improve diagnosis and treatment of patients, e.g., by precisely detecting symptoms [3], evaluating biomarkers [4], or detecting pathogenic resistance or subtypes. [5,6] Furthermore, upcoming concepts such as federated learning [7,8] and swarm learning [9] allow the cross-clinical creation of data-driven models without disrupting patient’s privacy [10-11], opening the gate for more powerful data-driven development. | |||
However, software has its risks, especially within [[medical device]]s. To protect patients from any risk of injury, disability, or other harmful interventions, medical device software (MDSW)—which is intended to provide specific medical purposes, such as diagnosis, monitoring, prognosis, or treatment—are subject to strict regulations. Examples include the European Medical Devices Regulation (MDR) [12], the In Vitro Diagnostic Medical Devices Regulation (IVDR) [13], and the International Medical Device Regulators Forum (IMDRF). [14] | |||
MDSW can be an integral part of a medical product or standalone software as an independent medical device. [15] MDSW can run in the [[Cloud computing|cloud]], as part of a software platform, or on a server, with both healthcare professionals and laypersons using it. Exceptions are software tools used for documentation or that solely control medical device hardware, or that serve no medical purpose. [13] | |||
An integral part of all regulations for MDSW is development according to the software life cycle process, as defined by [[IEC 62304]] [16], a harmonized international standard that regulates MDSW life cycle development, requiring documentation and processes, such as software development planning, requirement analysis, architectural design, testing, verification, and maintenance. [12,17] | |||
These regulations focus on minimizing patient risk, for example, harmful follow-up analysis, a wrong or missing treatment where needed, as a result of software failures, incorrect predictions, or other malfunctions. Several challenges arise in the attempt to eliminate these risks and allow for a smoother transfer of technology and greater reproducibility. | |||
===Challenges of scientific software development for health care=== | |||
The primary goal of scientists remains conducting scientific activities rather than developing software. However, many scientists aim to make their findings and methodologies available to a broader audience and to be used for the greater good. Thus, many data science and AI methodologies, as well as corresponding implementations and software packages, exist that would, in theory, allow the development of efficacious AI-driven CDSSs and MDSW. However, scientists of different backgrounds tend to have very different knowledge of software engineering practices, often acquired through self-study. [18] Moreover, academic groups often consist of small teams that undergo frequent change or researchers that work on a "one person-one project" basis. [19,20] Thus, a lack of attention to relevant software development processes and engineering practices defined by the software life cycle negatively affects the usefulness of developed packages, particularly for developing software as a medical device. [21] | |||
Pinning down the most critical requirements along with an accurate description and documentation of such is a significant challenge for all software projects independent of if it is conducted in research or industry. [18,22] It necessitates a detailed analysis of the non-functional requirements as determined, for instance, by regulatory entities, addressing aspects such as [[Software development security|security]], [[Information privacy|privacy]], or infrastructural limitations, as well as functional requirements like user-friendly interfaces and specific results. This is very time-consuming and relies on a close interaction of developers, stakeholders, and potential users, which is often difficult to achieve under academic circumstances. [20,22] Moreover, researchers are enticed by academic hiring procedures and driven by funders to focus on “novelty” rather than [[software quality]] and practical usefulness. [19,20] Thus, implementations often fail to fulfill requirements, ensuring long-term sustainability such as documentation, usability, appropriate performance for practical application, user-friendliness optimally supporting potential users, and minimizing risks. [19,23] | |||
The most critical aspects of ensuring sustainability in academic software are reproducibility, reusability, and traceability. [21,24] However, it has been shown that not only public accessibility but also documentation and portability are essential to ensure reproducibility and underpin trust in the scientific record of scientific software, enabling the re-use of research and code. Moreover, the prototype-centered development procedures often lack quality checks, such as systematic testing, that would ensure reusability. [21] Recently, scientific journals such as ''GigaScience'' or ''Biostatistics'' have promoted reproducibility and reusability by mandating the FAIR Principles (i.e., findability, accessibility, interoperability, and reusability). FAIR establishes a guideline for scientific [[Information management|data management]] and documentation. [19,25] Implementing the FAIR principles in academic software development has the potentil to lower the barriers to a successful industrial transition. | |||
Additionally, for long-term software maintenance, well-structured development planning and processes can ensure the traceability of modifications via [[change management]] and [[version control]]. These aspects ultimately determine scientific rigor, transparency, and reproducibility. [19] | |||
===Software life cycle=== | |||
Proper implementation of the software life cycle (SLC) guarantees high-quality planning, development, and maintenance of MDSW. The SLC deals with the planning and specification, development, maintenance, and configuration of the software. IEC 62304 defines the SLC as a conceptual structure across its lifetime, from the requirements’ definition to final release. It describes processes, tasks, and activities involved in developing a software product and their order and interdependencies. Furthermore, it defines milestones verifying the completeness of the results to be delivered. [16] | |||
However, a complete SLC described in standards like IEC 62304 is not feasible for most research projects. [26] Academic research is often subject to tight schedules and focuses on proof-of-concept development, neglecting formal documentation or procedures. Here we provide recommendations for an SLC in academia, lowering the boundary for many research organizations to implement an SLC and fostering the transfer of technology to industrial development. [13,26] | |||
===Our goal: An academia-tailored software life cycle=== | |||
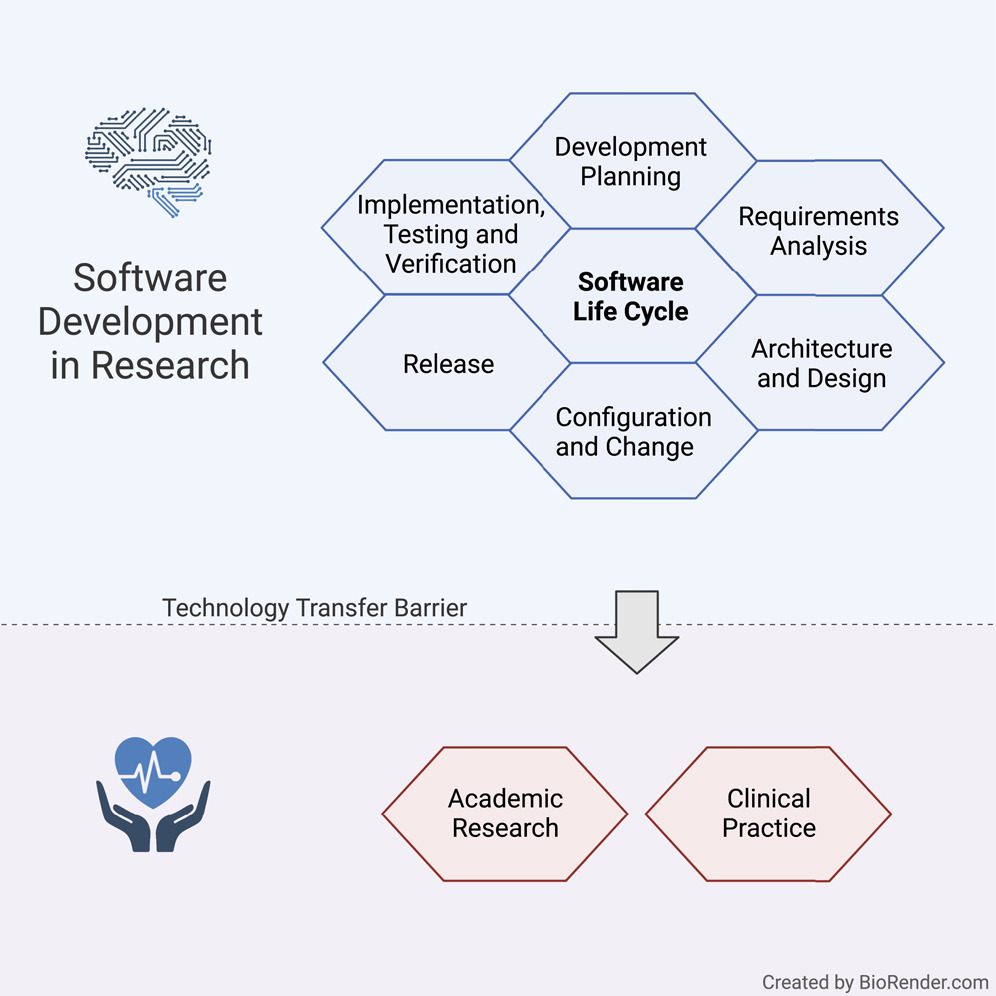
Until now, there has been little guidance on supporting a structured software development culture for academic institutions according to standard SLC processes. In this article, we present a synopsis of all requirements in official standards that are relevant to academia. We adjusted these toward the specific demands of software development in research and established a limited SLC process for research organizations, which has the potential to greatly facilitate and speed up such technology transfer and reproducibility in a controlled and predictable way. Being aware that a complete SLC is not feasible for most academic settings, we proposed a subset of elements that we are convinced will provide a significant benefit without creating an excessive organizational burden for researchers and developers and keep activities in a manageable range. | |||
Our proposal is centered on procedures for software development planning, software requirement analysis, software architectural design, software unit implementation, integration, [[Software reliability testing|testing]], [[Software verification and validation|verification]], and configuration management. Depending on the specific needs, the elements of an SLC process that work best for an organization may differ from what we propose. The fact, however, that a life cycle process is set up at all and that the elements are deliberately chosen is probably a key factor for facilitating technology transfer. However, any medical software development for clinical use must strictly follow the regulations relevant to the specific country or region. Thus, our guideline can only provide a starting point intended to be adapted to institute- or project-specific requirements, considering only relevant aspects. Nevertheless, the ideas presented here are not meant to provide a shortcut for medical software. An overview of our suggested SLC activities in tandem with regulations is provided in Table S1 of the Supplemental information. | |||
==Software life cycle for medical software research guideline== | |||
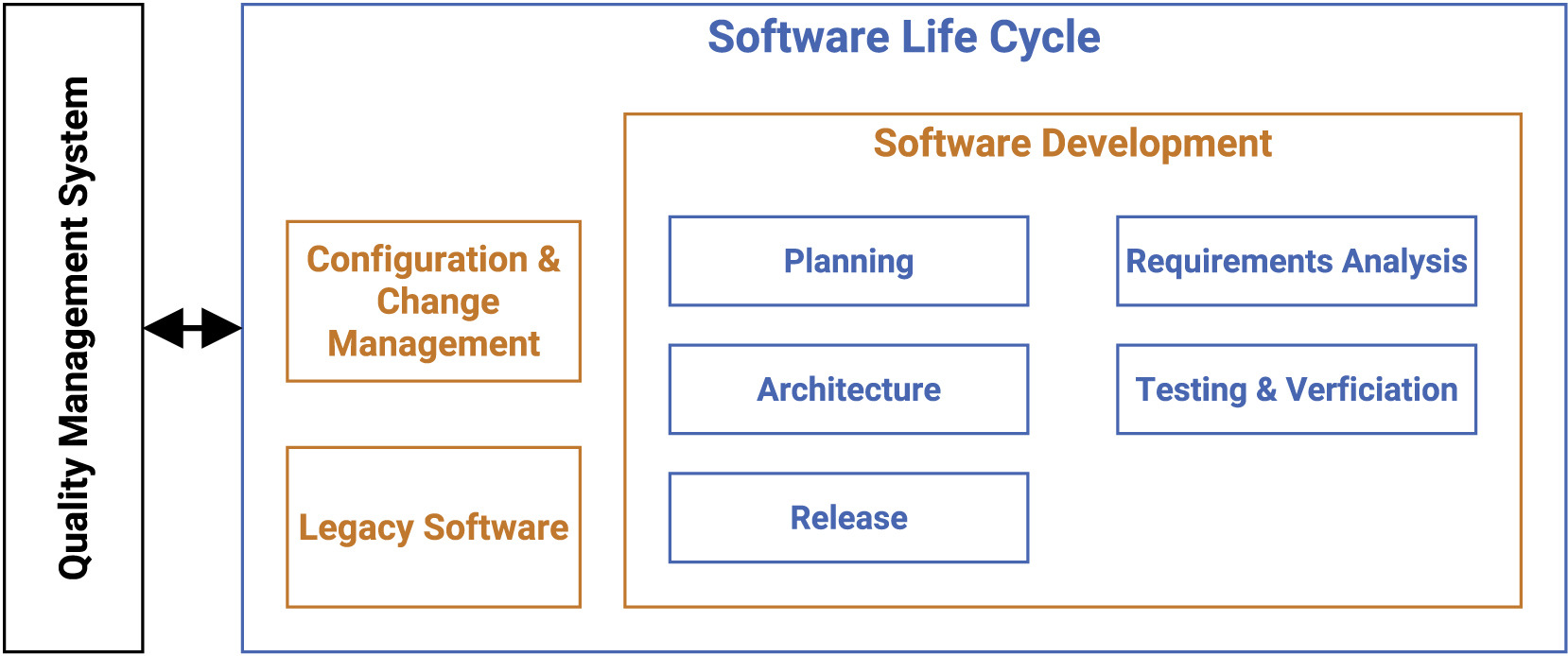
Our guideline covers multiple processes such as development planning, requirement analysis, software architecture, software design, implementation, software, and integration testing, verification, and release. In the following, we present the most vital points for the SLC, as depicted in Figure 1. | |||
[[File:Fig1 Hauschild iScience2022 25-12.jpg|800px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="800px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Figure 1.''' Main components of the software life cycle (SLC) for research-based medical software.</blockquote> | |||
|- | |||
|} | |||
|} | |||
The main focus of the SLC, in compliance with the [[quality management system]] (QMS), lies in the software development arena, while also tapping into configuration and change management, as well as legacy software. | |||
===Software development: Planning=== | |||
Defining an accurate software development plan is the first key component and must be updated regularly during the project, getting referenced and refined throughout the entire software life cycle model. It defines norms, methods, used processes, deliverable results, traceability between requirements, software testing, implemented risk control measures, configuration and change management, and verification of configuration elements, including software of unknown provenance (SOUP). (SOUP is a commonly used basic software library or set of packages that have not been developed for medical purposes.) | |||
Two documents should be provided for the academic-tailored implementation: the process description and the development plan. Each document produced during software development has to contain a title, purpose, and responsible person. [16] In the case of multiple involved developers, the role and responsibility assignments should be noted down in the single process description. | |||
First, the process description is provided by the definition of standard operating procedures (SOPs) for repetitive application problems. [27] The description contains each activity within the processes, when it will be completed, by whom, how, and with which input and output. The developed process description can be applied to multiple projects and has to be implemented by the developers. [12] Since the life cycle model must be completely defined or referenced by the development plan, a software engineering model must be selected to provide a general structure for the development phase, such as the V-model. As a common approach in medical device development, the V-model is successfully used to achieve [[regulatory compliance]]. [28] Generally, the selected model should match the project’s characteristics and thus can host agile practices or elements of SCRUM to support and conform to life cycle development practices. [29,30] | |||
Second, the development plan describes the general documentation required for a product or project. The development plan defines concrete milestones to corresponding deadlines, assigns designated staff to the pre-defined roles, and refines measures or tools adjusted to the project. It is challenging to meet the regulatory requirement of defining tools, testing, and configuration management in advance, particularly in a volatile academic setting. Due to external factors and unforeseeable changes, the development plan must be updated over the lifetime of the project, while the pre-defined process descriptions remain. Additionally, the process should recommend defining a coding guideline or convention, including code style, nomenclature, and naming in the development plan, to increase software quality. For example, using pre-defined rules in git hooks or continuous integration (CI), combined with linting tools, can enforce coding compliance. | |||
The development process gets more transparent and well-structured through the provision of these two documents. | |||
===Software development: Requirements analysis=== | |||
Revision as of 17:06, 4 July 2023
| Full article title | Guideline for software life cycle in health informatics |
|---|---|
| Journal | iScience |
| Author(s) | Hauschild, Anne-Christin; Martin, Roman; Holst, Sabrina C.; Wienbeck, Joachim; Heider, Dominik |
| Author affiliation(s) | Philipps University of Marburg, University Medical Center Göttingen |
| Primary contact | Email: dominik dot heider at uni-marburg dot de |
| Year published | 2022 |
| Volume and issue | 25(12) |
| Article # | 105534 |
| DOI | 10.1016/j.isci.2022.105534 |
| ISSN | 2589-0042 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.sciencedirect.com/science/article/pii/S2589004222018065 |
| Download | https://www.sciencedirect.com/science/article/pii/S2589004222018065/pdfft (PDF) |
Abstract
The long-lasting trend of medical informatics is to adapt novel technologies in the medical context. In particular, incorporating artificial intelligence (AI) to support clinical decision-making can significantly improve monitoring, diagnostics, and prognostics for the patient’s and medic’s sake. However, obstacles hinder a timely technology transfer from research to the clinic. Due to the pressure for novelty in the research context, projects rarely implement quality standards.
Here, we propose a guideline for academic software life cycle processes tailored to the needs and capabilities of research organizations. While the complete implementation of a software life cycle according to commercial standards is not feasible in scientific work, we propose a subset of elements that we are convinced will provide a significant benefit while keeping the effort within a feasible range.
Ultimately, the emerging quality checks for academic software development can pave the way for an accelerated deployment of academic advances in clinical practice.
Keywords: health informatics, bioinformatics, software engineering
Introduction
Today, medical informatics is an integral part of health care systems that ensure the smooth operation of processes in medical care. Moreover, standard procedures ensure the transfer of knowledge from medical research to clinical practice, for instance, via regularly updated guidelines and regulations. In contrast, newly developed software innovations—such as systems based on artificial intelligence (AI) that could support clinical decisions and have already evolved to be the state-of-the-art in medical informatics research—rarely transfer to application in practice.
Modern methods such as AI and machine learning (ML) increasingly unroll their potential in medical healthcare to help patients and clinicians. [1] Clinical decision support systems (CDSSs) can effectively increase diagnostics, patient safety, and cost containment. [2] Easily accessible AI-based applications can improve diagnosis and treatment of patients, e.g., by precisely detecting symptoms [3], evaluating biomarkers [4], or detecting pathogenic resistance or subtypes. [5,6] Furthermore, upcoming concepts such as federated learning [7,8] and swarm learning [9] allow the cross-clinical creation of data-driven models without disrupting patient’s privacy [10-11], opening the gate for more powerful data-driven development.
However, software has its risks, especially within medical devices. To protect patients from any risk of injury, disability, or other harmful interventions, medical device software (MDSW)—which is intended to provide specific medical purposes, such as diagnosis, monitoring, prognosis, or treatment—are subject to strict regulations. Examples include the European Medical Devices Regulation (MDR) [12], the In Vitro Diagnostic Medical Devices Regulation (IVDR) [13], and the International Medical Device Regulators Forum (IMDRF). [14]
MDSW can be an integral part of a medical product or standalone software as an independent medical device. [15] MDSW can run in the cloud, as part of a software platform, or on a server, with both healthcare professionals and laypersons using it. Exceptions are software tools used for documentation or that solely control medical device hardware, or that serve no medical purpose. [13]
An integral part of all regulations for MDSW is development according to the software life cycle process, as defined by IEC 62304 [16], a harmonized international standard that regulates MDSW life cycle development, requiring documentation and processes, such as software development planning, requirement analysis, architectural design, testing, verification, and maintenance. [12,17]
These regulations focus on minimizing patient risk, for example, harmful follow-up analysis, a wrong or missing treatment where needed, as a result of software failures, incorrect predictions, or other malfunctions. Several challenges arise in the attempt to eliminate these risks and allow for a smoother transfer of technology and greater reproducibility.
Challenges of scientific software development for health care
The primary goal of scientists remains conducting scientific activities rather than developing software. However, many scientists aim to make their findings and methodologies available to a broader audience and to be used for the greater good. Thus, many data science and AI methodologies, as well as corresponding implementations and software packages, exist that would, in theory, allow the development of efficacious AI-driven CDSSs and MDSW. However, scientists of different backgrounds tend to have very different knowledge of software engineering practices, often acquired through self-study. [18] Moreover, academic groups often consist of small teams that undergo frequent change or researchers that work on a "one person-one project" basis. [19,20] Thus, a lack of attention to relevant software development processes and engineering practices defined by the software life cycle negatively affects the usefulness of developed packages, particularly for developing software as a medical device. [21]
Pinning down the most critical requirements along with an accurate description and documentation of such is a significant challenge for all software projects independent of if it is conducted in research or industry. [18,22] It necessitates a detailed analysis of the non-functional requirements as determined, for instance, by regulatory entities, addressing aspects such as security, privacy, or infrastructural limitations, as well as functional requirements like user-friendly interfaces and specific results. This is very time-consuming and relies on a close interaction of developers, stakeholders, and potential users, which is often difficult to achieve under academic circumstances. [20,22] Moreover, researchers are enticed by academic hiring procedures and driven by funders to focus on “novelty” rather than software quality and practical usefulness. [19,20] Thus, implementations often fail to fulfill requirements, ensuring long-term sustainability such as documentation, usability, appropriate performance for practical application, user-friendliness optimally supporting potential users, and minimizing risks. [19,23]
The most critical aspects of ensuring sustainability in academic software are reproducibility, reusability, and traceability. [21,24] However, it has been shown that not only public accessibility but also documentation and portability are essential to ensure reproducibility and underpin trust in the scientific record of scientific software, enabling the re-use of research and code. Moreover, the prototype-centered development procedures often lack quality checks, such as systematic testing, that would ensure reusability. [21] Recently, scientific journals such as GigaScience or Biostatistics have promoted reproducibility and reusability by mandating the FAIR Principles (i.e., findability, accessibility, interoperability, and reusability). FAIR establishes a guideline for scientific data management and documentation. [19,25] Implementing the FAIR principles in academic software development has the potentil to lower the barriers to a successful industrial transition.
Additionally, for long-term software maintenance, well-structured development planning and processes can ensure the traceability of modifications via change management and version control. These aspects ultimately determine scientific rigor, transparency, and reproducibility. [19]
Software life cycle
Proper implementation of the software life cycle (SLC) guarantees high-quality planning, development, and maintenance of MDSW. The SLC deals with the planning and specification, development, maintenance, and configuration of the software. IEC 62304 defines the SLC as a conceptual structure across its lifetime, from the requirements’ definition to final release. It describes processes, tasks, and activities involved in developing a software product and their order and interdependencies. Furthermore, it defines milestones verifying the completeness of the results to be delivered. [16]
However, a complete SLC described in standards like IEC 62304 is not feasible for most research projects. [26] Academic research is often subject to tight schedules and focuses on proof-of-concept development, neglecting formal documentation or procedures. Here we provide recommendations for an SLC in academia, lowering the boundary for many research organizations to implement an SLC and fostering the transfer of technology to industrial development. [13,26]
Our goal: An academia-tailored software life cycle
Until now, there has been little guidance on supporting a structured software development culture for academic institutions according to standard SLC processes. In this article, we present a synopsis of all requirements in official standards that are relevant to academia. We adjusted these toward the specific demands of software development in research and established a limited SLC process for research organizations, which has the potential to greatly facilitate and speed up such technology transfer and reproducibility in a controlled and predictable way. Being aware that a complete SLC is not feasible for most academic settings, we proposed a subset of elements that we are convinced will provide a significant benefit without creating an excessive organizational burden for researchers and developers and keep activities in a manageable range.
Our proposal is centered on procedures for software development planning, software requirement analysis, software architectural design, software unit implementation, integration, testing, verification, and configuration management. Depending on the specific needs, the elements of an SLC process that work best for an organization may differ from what we propose. The fact, however, that a life cycle process is set up at all and that the elements are deliberately chosen is probably a key factor for facilitating technology transfer. However, any medical software development for clinical use must strictly follow the regulations relevant to the specific country or region. Thus, our guideline can only provide a starting point intended to be adapted to institute- or project-specific requirements, considering only relevant aspects. Nevertheless, the ideas presented here are not meant to provide a shortcut for medical software. An overview of our suggested SLC activities in tandem with regulations is provided in Table S1 of the Supplemental information.
Software life cycle for medical software research guideline
Our guideline covers multiple processes such as development planning, requirement analysis, software architecture, software design, implementation, software, and integration testing, verification, and release. In the following, we present the most vital points for the SLC, as depicted in Figure 1.
|
The main focus of the SLC, in compliance with the quality management system (QMS), lies in the software development arena, while also tapping into configuration and change management, as well as legacy software.
Software development: Planning
Defining an accurate software development plan is the first key component and must be updated regularly during the project, getting referenced and refined throughout the entire software life cycle model. It defines norms, methods, used processes, deliverable results, traceability between requirements, software testing, implemented risk control measures, configuration and change management, and verification of configuration elements, including software of unknown provenance (SOUP). (SOUP is a commonly used basic software library or set of packages that have not been developed for medical purposes.)
Two documents should be provided for the academic-tailored implementation: the process description and the development plan. Each document produced during software development has to contain a title, purpose, and responsible person. [16] In the case of multiple involved developers, the role and responsibility assignments should be noted down in the single process description.
First, the process description is provided by the definition of standard operating procedures (SOPs) for repetitive application problems. [27] The description contains each activity within the processes, when it will be completed, by whom, how, and with which input and output. The developed process description can be applied to multiple projects and has to be implemented by the developers. [12] Since the life cycle model must be completely defined or referenced by the development plan, a software engineering model must be selected to provide a general structure for the development phase, such as the V-model. As a common approach in medical device development, the V-model is successfully used to achieve regulatory compliance. [28] Generally, the selected model should match the project’s characteristics and thus can host agile practices or elements of SCRUM to support and conform to life cycle development practices. [29,30]
Second, the development plan describes the general documentation required for a product or project. The development plan defines concrete milestones to corresponding deadlines, assigns designated staff to the pre-defined roles, and refines measures or tools adjusted to the project. It is challenging to meet the regulatory requirement of defining tools, testing, and configuration management in advance, particularly in a volatile academic setting. Due to external factors and unforeseeable changes, the development plan must be updated over the lifetime of the project, while the pre-defined process descriptions remain. Additionally, the process should recommend defining a coding guideline or convention, including code style, nomenclature, and naming in the development plan, to increase software quality. For example, using pre-defined rules in git hooks or continuous integration (CI), combined with linting tools, can enforce coding compliance.
The development process gets more transparent and well-structured through the provision of these two documents.
Software development: Requirements analysis
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation, though grammar and word usage was substantially updated for improved readability. In some cases important information was missing from the references, and that information was added.