Journal:Laboratory diagnosis of COVID-19 in China: A review of challenging cases and analysis
| Full article title | Laboratory diagnosis of COVID-19 in China: A review of challenging cases and analysis |
|---|---|
| Journal | Journal of Microbiology, Immunology and Infection |
| Author(s) | Jing, Ran; Kudinha, Timothy; Zhou, Meng-Lan; Xiao, Meng; Wang, He; Yang, Wen-Hang; Xu, Ying-Chun; Hsueh, Po-Ren |
| Author affiliation(s) |
Chinese Academy of Medical Sciences, Beijing Key Laboratory for Mechanisms Research and Precision Diagnosis of Invasive Fungal Diseases, Charles Sturt University, NSW Health Pathology, National Taiwan University College of Medicine |
| Primary contact | Email: tkudinha at yahoo dot com |
| Year published | 2020 |
| Volume and issue | In Press |
| DOI | 10.1016/j.jmii.2020.10.004 |
| ISSN | 1684-1182 |
| Distribution license | Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International |
| Website | https://www.sciencedirect.com/science/article/pii/S1684118220302498 |
| Download | https://www.sciencedirect.com/science/article/pii/S1684118220302498/pdfft (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Since the initial emergence of coronavirus disease 2019 (COVID-19) in Wuhan, Hubei province, China, a rapid spread of the disease occurred around the world, becoming an international global health concern at the pandemic level. In the face of this medical challenge threatening humans, the development of rapid and accurate methods for early screening and diagnosis of COVID-19 became crucial to containing the emerging public health threat, and preventing further spread within the population. Despite the large number of COVID-19 confirmed cases in China, some problematic cases with inconsistent laboratory testing results were reported. Specifically, a high false-negative rate of 41% on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection by real-time reverse transcription polymerase chain reaction (qRT-PCR) assays was observed in China. Although serological testing has been applied worldwide as a complementary method to help identify SARS-CoV-2, several limitations on its use have been reported in China. Therefore, the separate use of qRT-PCR and serological testing in the diagnosis of COVID-19 in China and elsewhere presents considerable challenges, but when used in combination, these methods can be valuable tools in the fight against COVID-19. In this review, we give an overview of the advantages and disadvantages of different molecular techniques for SARS-CoV-2 detection that are currently used in several labs, including qRT-PCR, gene sequencing, loop-mediated isothermal amplification (LAMP), nucleic acid mass spectrometry (MS), and gene editing techniques based on the clustered regularly interspaced short palindromic repeats (CRISPR/Cas13) system. Then we mainly review and analyze some causes of false-negative qRT-PCR results, and how to resolve some of the diagnostic dilemmas.
Keywords: SARS-CoV-2, COVID-19, qRT-PCR, serology testing, challenging cases
Introduction
Soon after coronavirus disease 2019 (COVID-19) fully emerged in China at the beginning of 2020, the Chinese government immediately implemented strong measures to contain the outbreak. With great efforts, the COVID-19 cases have stabilized in China as a whole to date, albeit a small number of imported cases that intermittently emerge. However, an [[epidemic] began to rapidly spread around the world from April to date. As of August 21, 2020 (6:48pm CEST), there had been a total of 22,536,278 confirmed cases worldwide, with the largest cumulative number of COVID-19 confirmed cases (n = 5,477,305) in the United States of America (USA), followed by Brazil (n = 3,456,652), and India (n = 2.905,823).[1]
Some challenging cases of COVID-19 diagnosis were encountered in China and elsewhere, involving inconsistent laboratory testing results, mainly caused by false-negative real-time reverse transcription-polymerase chain reaction (qRT-PCR) detection. In this review, we summarize and discuss some possible causes of false-negative results, including how to resolve the diagnostic dilemma. We also review and discuss the advantages and disadvantages of the different lab assays for diagnosing COVID-19, including different molecular techniques and serological assays, and the value of combining qRT-PCR assays with serological testing. In brief, it is crucial to select appropriate diagnostic methods according to the phase of infection, or to use a combination of different methods and other clinical parameters in confirming the infection status of individuals.
SARS-CoV-2 etiological characteristics and genome organization
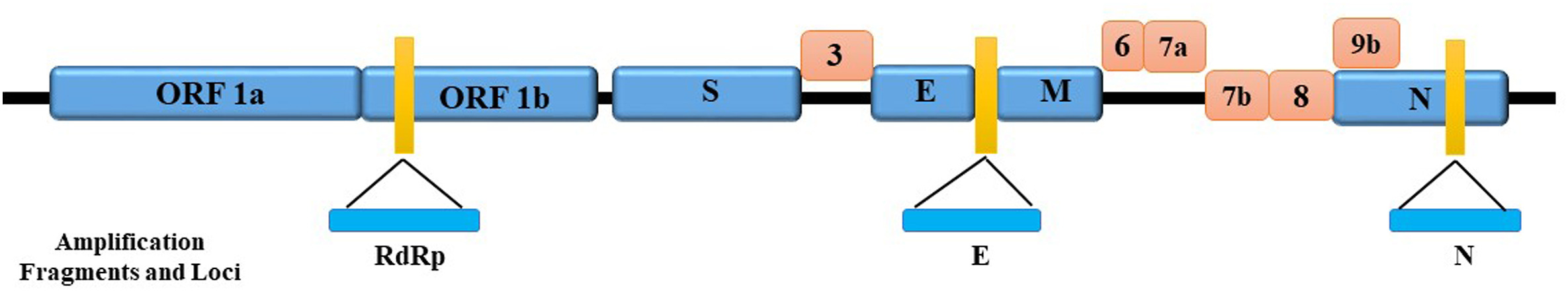
There are four genera under the subfamily coronavirus (CoVs)[2], including α, β, γ, and δ. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, responsible for COVID-19, belongs to the β CoV genus, the seventh member of the family of CoVs possessing a single-stranded[2][3], positive-sense RNA genome. The genome of the SARS-CoV-2 virus consists of about 29,000 bases.[2][4] Studies show that there are at least 12 coding regions, including open reading frames (ORF) 1 ab, S, 3, E, M, 7, 8, 9, 10b, N, 13, and 14.[4][5] Among them, ORF 1 ab is the region of the RdRp gene which codes for RNA polymerase and is responsible for viral nucleic acid replication.[6]
The structural proteins include[4]:
- a spike (S), crucially associated with virus transmission capacity, binding to angiotensin-converting enzyme 2 (ACE2) receptors on the cell surface to get into the host cell;
- an envelope protein (E), responsible for the formation of virus envelopes and virus particles;
- a membrane protein (M), responsible for membrane proteins encoded; and
- a nucleocapsid (N), having recognition with the host RNA of the virus genome.
These functional proteins play an essential role in genome maintenance and virus replication. Beyond these, several accessory proteins also help in virus replication, including ORF3, ORF6, ORF7a, ORF7b, ORF8, and ORF9b.[2] The amplication fragments and loci of genes coding these proteins are shown in Fig. 1.
|
Molecular diagnosis for COVID-19 confirmation
Real-time reverse transcription-polymerase chain reaction (qRT-PCR)
In many countries, the preferred testing method for COVID-19 confirmation is the qRT-PCR assay, which is regarded as the gold standard for virus infection confirmation. According to Diagnosis & Treatment Scheme for Coronavirus Disease 2019 (seventh edition, in Chinese), suspected COVID-19 cases are laboratory-confirmed for positive detection of SARS-CoV-2 RNA by qRT-PCR testing. This form of molecular testing offers several advantages in the diagnosis of COVID-19. Comparted to serology testing, qRT-PCR testing is much more valuable in the early phase of infection. Firstly, qRT-PCR results are generally available within a few hours, and the testing is easy to perform on a large scale, and with low cost per sample. However, high false-negative rates of SARS-CoV-2 detection have been reported in China (41%).[7]
Common qRT-PCR amplification fragments and loci of SARS-CoV-2 are shown in the prior Fig. 1. Different countries have selected different targets and designed different primers for qRT-PCR assays. The available primer and probe sequences designed by different countries are summarized in Table 1 below, including COVID-19 infection confirmatory tests for different qRT-PCR assays.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- ↑ World Health Oranization (2020). "WHO Coronavirus Disease (COVID-19) Dashboard". World Health Organization. https://covid19.who.int/table.
- ↑ 2.0 2.1 2.2 2.3 2.4 Chan, H. F.-W.; Kok, J.-H.; Zhu, Z. et al. (2020). "Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan". Emerging Microbes and Infections 9 (1): 221–36. doi:10.1080/22221751.2020.1719902. PMC PMC7067204. PMID 31987001. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7067204.
- ↑ 3.0 3.1 Zhu, N.; Zhang, D.; Wang, W. et al. (2020). "A Novel Coronavirus from Patients with Pneumonia in China, 2019". New England Journal of Medicine 382 (8): 727–33. doi:10.1056/NEJMoa2001017. PMC PMC7092803. PMID 31978945. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7092803.
- ↑ 4.0 4.1 4.2 4.3 Wang, X.; Shi, J.; Ding, W. et al. (2020). "Development of research and application of nucleic acid detection of 2019 novel coronavirus". Chinese Journal of Clinical Laboratory Science 38: 81–84. doi:10.13602/j.cnki.jcls.2020.02.01.
- ↑ Lu, R.; Zhao, X.; Li, J. et al. (2020). "Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding". Lancet 395 (10224): 565–74. doi:10.1016/S0140-6736(20)30251-8. PMC PMC7159086. PMID 32007145. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7159086.
- ↑ Huang, C.; Wang, Y.; Li, X. et al. (2020). "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China". Lancet 395 (10223): 497–506. doi:10.1016/S0140-6736(20)30183-5. PMC PMC7159299. PMID 31986264. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7159299.
- ↑ Ai, T.; Yang, Z.; Hou, H. et al. (2020). "Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases". Radiology 296 (2): E32–40. doi:10.1148/radiol.2020200642. PMC PMC7233399. PMID 32101510. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7233399.
- ↑ National Health Commission of the People’s Republic of China (21 February 2020). "Technical Guide for Prevention and Control of Coronavirus Disease 2019 in Medical Institutions, 5th Edition". http://www.nhc.gov.cn/jkj/s3577/202002/a5d6f7b8c48c451c87dba14889b30147.shtml.
- ↑ 9.0 9.1 Corman, V.M.; Landt, O.; Kaiser, M. et al. (2020). "Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR". Euro Surveillance 25 (3): 2000045. doi:10.2807/1560-7917.ES.2020.25.3.2000045. PMC PMC6988269. PMID 31992387. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6988269.
- ↑ 10.0 10.1 World Health Organization (24 January 2020). "Molecular assays to diagnose COVID-19: Summary table of available protocols". World Health Organization. https://www.who.int/publications/m/item/molecular-assays-to-diagnose-covid-19-summary-table-of-available-protocols.
- ↑ U.S. Department of Health and Human Services (29 May 2020). "2019-Novel Coronavirus (2019-nCoV) Real-time rRT-PCR Panel Primers and Probes" (PDF). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/downloads/rt-pcr-panel-primer-probes.pdf.
- ↑ University of Hong Kong, School of Public Health (2020). "Detection of 2019 novel coronavirus (2019-nCoV) in suspected human cases by RT-PCR" (PDF). World Health Organization. https://www.who.int/docs/default-source/coronaviruse/peiris-protocol-16-1-20.pdf.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation, spelling, and grammar. In some cases important information was missing from the references, and that information was added. Otherwise, no other changes were made in conformance with the "NoDerivatives" portion of the document license.