Journal:Management of post-analytical processes in the clinical laboratory according to ISO 15189:2012: Considerations about the management of clinical samples, ensuring quality of post-analytical processes and laboratory information management
| Full article title | Management of post-analytical processes in the clinical laboratory according to ISO 15189:2012: Considerations about the management of clinical samples, ensuring quality of post-analytical processes and laboratory information management |
|---|---|
| Journal | Advances in Laboratory Medicine |
| Author(s) | Yeste, Mᵃ L.L.; Mas, Antonia R.P.; Muñoz, Leonor G.; Álvarez, Silvia I.; García, Fernando M.; Font, Aurora B.; Gómez, Natalia F.P.; Gancedo, Lorena S.; Álvarez, Ana G.; Andreu, Francisco A.B.; Rodríguez, Mᵃ P.C.; Domínguez, Luisa Á. |
| Author affiliation(s) | CATLAB, Hospital Universitari Son Espases, Hospital de la Santa Creu y Sant Pau, Hospital Universitario Miguel Servet, Germans Trias i Pujols Universitary Hospital, Hospital Universitari de Bellvitge, Hospital Universitario de la Princesa, Institute of Oncologic and Molecular Oncology, Hospital Clínico San Carlos, Hospital Universitario Puerta de Hierro, Laboratory Accreditation Board of the Spanish Society of Laboratory Medicine |
| Primary contact | Email: llopez at catlab dot cat |
| Year published | 2021 |
| Volume and issue | 2(3) |
| Page(s) | 373-380 |
| DOI | 10.1515/almed-2021-0044 |
| ISSN | 2628-491X |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.degruyter.com/document/doi/10.1515/almed-2021-0044/html |
| Download | https://www.degruyter.com/document/doi/10.1515/almed-2021-0044/pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
ISO 15189:2012 Medical laboratories — Requirements for quality and competence establishes the requirements for clinical specimen management, ensuring the quality of processes and laboratory information management. ENAC (Entidad Nacional de Acreditación), the sole accreditation authority in Spain, established the requirements for the authorized use of the ISO 15189 accreditation label in reports issued by accredited laboratories. These recommendations are applicable to the lab's post-analytical processes and the professionals involved. The standard requires laboratories to define and document the duration and conditions of specimen retention. Laboratories are also required to design an internal quality control scheme to verify whether post-analytical activities attain the expected standards. Information management requirements are also established, and laboratories are required to design a contingency plan to ensure the communication of laboratory results. Instructions are finally provided about the correct use of the accreditation label in laboratory reports. A range of nations and scientific societies support clinical laboratories being required to obtain accreditation. With ISO 15189 being the most specific standard for demonstrating technical performance, a clear understanding of its requirements is essential for proper implementation.
Keywords: accreditation, clinical laboratory, ISO 15189 standard, laboratory information system, post-analytical
Introduction
Clinical laboratories increasingly devote more efforts to improving their methodological and communication skills to help physicians in the interpretation of test results and improve patient outcomes. This type of quality control is found in UNE EN ISO 15189:2013 Medical laboratories — Requirements for quality and competence (ISO 15189:2012, Corrected version 2014-08-15; hereinafter, the ISO 15189 standard), which addresses the revision, reporting, and release of clinical test results[1], as well as other requirements for post-analytical processes. These requirements also address specimen storage, retention, and disposal; the inclusion of post-analytical processes in laboratory quality assurance and continuous improvement; laboratory information management; and the need for a contingency plan that ensures the communication of test results in any scenario.[2][3] ISO 15189 is based on laboratory best practices and employs the information obtained from the lab's quality management system to generate corrective and improvement actions.
ISO 15189 also establishes a set of requirements for laboratories to implement effective methods for the detection and classification of post-analytical errors and the incorporation of information systems and standard operating procedures (SOPs) aimed at reducing errors.[4] Special emphasis is placed on the communication of results, laboratory information management, and risk management. The standard also requires that a contingency plan is designed.
Additionally, and considering that the use of the ENAC (Entidad Nacional de Acreditación) label in laboratory reports is the way Spanish laboratories demonstrate that they comply with accreditation requirements, it is important all accredited laboratories pay attention to the ENAC document CEA-ENAC-01 Requirements for the use of the ENAC label and reference of certification, which establishes the requirements for ENAC and ISO 15189 accreditation label use.[5] However, these ENAC recommendations do not extend the standard and should be considered complementary information that facilitates its interpretation and implementation. The scope of application is the staff involved in post-analytical processes in the clinical laboratory.
Specimen storage, retention, and disposal
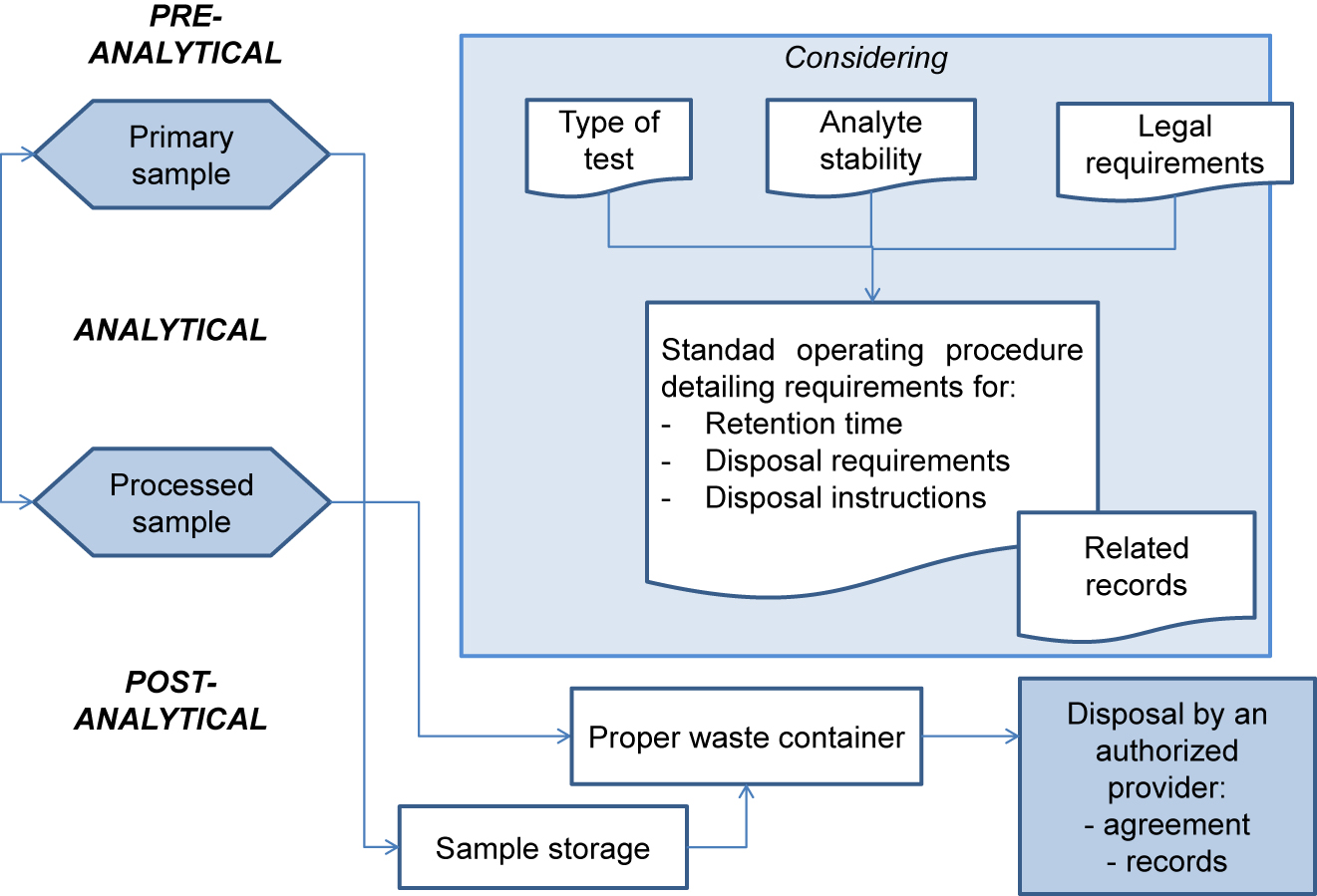
ISO 15189 requires that the laboratory define and document the duration of specimen retention, as well as specimen storage and discard conditions. The standard operating procedure should specify the duration of specimen retention and storage conditions, which will be defined according to the nature and stability of each analyte[6] and the applicable legal requirements (see Figure 1). The duration of specimen storage may be extended by legal requirements associated with some types of studies (e.g., histological analyses, gene testing, pediatric studies).
|
Access to archive specimens must be restricted. Easy specimen traceability and location for retrieval must be ensured (for additional testing, result verification, and/or legal requirement, among others). Therefore, it is recommended that the date and person responsible for specimen retrieval be recorded, which is essential in case it is required by law.
The procedure for disposal of clinical specimens and consumables must be documented by the laboratory. It is laboratory’s responsibility to comply with the laws and regulations in relation to the prevention of occupation hazards, even though waste disposal is performed by an authorized external supplier.
Quality assurance and continuous improvement
As with pre-analytical and analytical procedures, post-analytical procedures must be evaluated and audited to ensure compliance with standards. Auditing is also intended to verify whether the process satisfies user’s needs and requirements. Thus, efforts are necessary to improve the effectiveness of the post-analytical process.
References
- ↑ López Yeste, Ma Liboria; Izquierdo Álvarez, Silvia; Pons Mas, Antonia R.; Álvarez Domínguez, Luisa; Blanco Font, Aurora; Marqués García, Fernando; Bernabeu Andreu, Francisco A.; Rodríguez, Ma Patrocinio Chueca et al. (10 March 2021). "Gestión del proceso posanalítico en los laboratorios clínicos según los requisitos de la norma ISO 15189:2012. Consideraciones sobre la revisión, notificación y comunicación de los resultados" (in en). Advances in Laboratory Medicine / Avances en Medicina de Laboratorio 2 (1): 61–70. doi:10.1515/almed-2020-0027. ISSN 2628-491X. https://www.degruyter.com/document/doi/10.1515/almed-2020-0027/html.
- ↑ "UNE-EN ISO 15189:2013". Tienda AENOR. AENOR. 12 June 2013. https://tienda.aenor.com/norma-une-en-iso-15189-2013-n0051322.
- ↑ "CGA-ENAC-LCL Rev. 3 Criterios generales de acreditación de Laboratorios Clínicos" (PDF). ENAC. September 2018. https://www.enac.es/documents/7020/b569e184-a2aa-4dcd-88a5-1d1ceb9033db.
- ↑ Beastall, Graham H. (1 January 2013). "Adding value to laboratory medicine: a professional responsibility" (in en). Clinical Chemistry and Laboratory Medicine (CCLM) 51 (1): 221–227. doi:10.1515/cclm-2012-0630. ISSN 1437-4331. https://www.degruyter.com/document/doi/10.1515/cclm-2012-0630/html.
- ↑ "CEA-ENAC-01 Criterios para la utilización de la marca ENAC o referencia a la condición de acreditado" (PDF). ENAC. April 2020. https://www.enac.es/documents/7020/88f9773a-6214-45ef-9618-3b7efc549699.
- ↑ Alsina, M.J.; Álvarez, V.; Bueno, M. et al. (2006). "Protocolo para el estudio de la estabilidad de las magnitudes biológicas". Química Clínica 25 (2): 86–89. https://www.seqc.es/download/doc/21/2796/759467396/69509/cms/protocolo-para-el-estudio-de-la-estabilidad-de-las-magnitudes-biologicas-2006.pdf/. Retrieved 16 November 2020.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation, spelling, and grammar. In some cases important information was missing from the references, and that information was added.