Journal:Quality management system implementation in human and animal laboratories
| Full article title | Quality management system implementation in human and animal laboratories |
|---|---|
| Journal | One Health |
| Author(s) | Kachuwaire, Obert; Zakaryan, Arsen; Manjengwa, Julius; Davtyan, Zaruhi; Châtard, Jerome; Orelle, Arnaud; Tumanyan, Pertch; Petikyan, Aida; Hambardzumyan, Nune; Pierson, Antoine |
| Author affiliation(s) | Integrated Quality Laboratory Services, Republican Veterinary-Sanitary and Phytosanitary Laboratory Services Center, Armenian Centers for Disease Control and Prevention |
| Primary contact | Email: kachuwaire at iqls dot net |
| Year published | 2021 |
| Volume and issue | 13 |
| Article # | 100278 |
| DOI | 10.1016/j.onehlt.2021.100278 |
| ISSN | 2352-7714 |
| Distribution license | Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International |
| Website | https://www.sciencedirect.com/science/article/pii/S2352771421000689 |
| Download | https://www.sciencedirect.com/science/article/pii/S2352771421000689/pdfft (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Background: The ability to rapidly detect emerging and re-emerging threats relies on a strong network of laboratories providing high-quality testing services. Improving laboratory quality management systems (QMS) to ensure that these laboratories effectively play their critical role using a tailored stepwise approach can assist them to comply with World Health Organization's (WHO) International Health Regulations (IHRs), as well as the World Organization for Animal Health's (OIE) guidelines.
Methods: Fifteen (15) laboratories in Armenia's human and veterinary laboratory networks were enrolled into a QMS strengthening program from 2017 to 2020. Training was provided for key staff, resulting in an implementation plan developed to address gaps. Routine mentorship visits were conducted. Audits were undertaken at baseline and post-implementation using standardized checklists to assess laboratory improvements.
Results: Baseline audit general indicator scores ranged from 21% to 46% for human laboratories and 37% to 60% for the veterinary laboratories. Following implementation scores improved, ranging from 7% to 39% for human laboratories and 12% to 19% for veterinary laboratories.
Conclusion: In general, there has been improvement for both human and veterinary laboratories in the areas of QMS implementation, particularly in organizational structure, human resources, equipment management, supply chain management, and data management. Central facilities developed systems that are ready for international accreditation. This One Health strengthening project ensured simultaneous strengthening of both human and veterinary laboratories, which is not a common approach.
Keywords: laboratory quality management, One Health, laboratory assessments, veterinary laboratory, public health laboratory, standardization
Introduction
A core component within global health security initiatives, including the International Health Regulations (IHRs) and Global Health Security Agenda (GHSA), is the need for responsive and technically competent laboratories.[1][2][3][4] These laboratories play a frontline role in disease detection, surveillance, and response efforts, especially crucial in light of threats from emerging and re-emerging infections of pandemic potential[5], like the current COVID-19 response. Laboratory results and data generated from these entities are useful if they are reliable and reproducible, eliciting trust and confidence in end users.[6] However, in many low- and middle-income countries (LMICs), laboratory quality standards are hampered by a myriad of factors, including lack of regulations, scarce resources and expertise to set up such systems, and the high cost of international accreditation programs.[1]
Laboratory strengthening efforts that incorporate stepwise implementation of quality management systems (QMS) have been promoted globally since the WHO 2008 Lyon meeting on quality, and the subsequent key global calls to action.[3][7] An adequate nationwide laboratory system that is able to reliably support outbreak and surveillance activities consists of human health laboratories, among other sectors, including animal, food, water, and environmental health (depending on the context), as most of the emerging and re-emerging disease threats are proving to be zoonotic.[8][9][10] Therefore, a QMS implementation strategy that seeks to not just enable human health laboratories but also leverage a One Health approach—where "multiple sectors communicate and work together to achieve better public health outcomes"[11]—is encouraged.
Quality assured diagnostics for both human and veterinary services are key in enhancing efficiencies in the government of Armenia’s (GoA) laboratory testing capacities to detect select agents at a minimal number of safe and secure facilities, as well as in enhancing safe, secure, and sustainable infectious disease surveillance and reporting.[12] In 2017, the GoA and the United States of America Defense Threat Reduction Agency (DTRA) collaborated in the strengthening of human and animal laboratories. They fall under the National Center for Disease Control (NCDCP) for human health and the Food Safety Inspectorate (FSI) for animal health. Through the International Science and Technology Center, Astana Kazakhstan, with Integrated Quality Laboratory Services (IQLS)—providing technical assistance—worked with the GoA and DTRA to address key gaps related to the laboratory QMS. Selected laboratories were composed of central facilities located in the capital city and satellite branches in regional locales (locally Marzes).
IQLS conducted laboratory assessments of both the human and animal laboratory networks. These assessments highlighted that a majority of laboratories were challenged in their QMS. In this article, we present three years of laboratory level QMS implementation (2017–2020). We describe a QMS strengthening approach that uses evidence-based results from laboratory system and on-site laboratory facility assessments to guide implementation, including training and on-site mentorship. We used adapted international tools, allowing for a phased approach as recommended for such settings.
Methods
Laboratory quality strengthening process
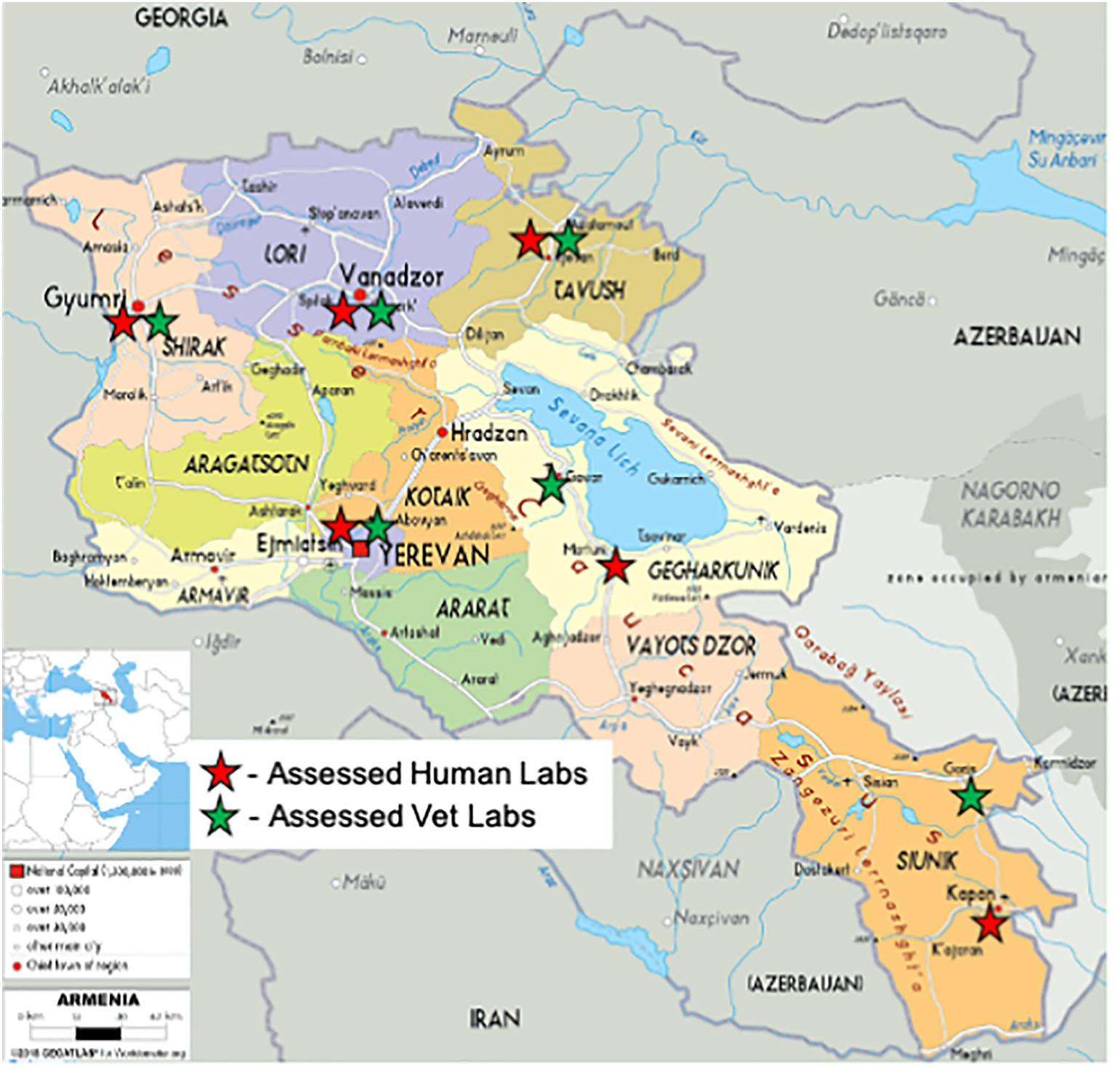
The baseline state of a QMS was determined through an inception period, consisting of system and site assessment of nine laboratories (human [n = 6] and animal health [n = 3]) in the fourth quarter of 2017 and early 2018. Information from these assessments was subsequently used for further site selection. Pursuant the inception period, fifteen (15) key laboratories that would form the backbone of the laboratory system were purposively selected by a joint GoA/DTRA working group and were composed of the following (see Figure 1):
- seven human health laboratories: Central Reference level (Yerevan) and its six Marz level branches
- two infectious disease hospital laboratories
- six Veterinary laboratories: Central Reference level and five Marz level laboratories
|
Two training sessions were organized (level one and two) to pass along knowledge and skills on QMS implementation. The level one workshop was designed as a two-week train-the-trainer model to assist laboratory quality managers and their deputies to develop competencies that are transferrable to other staff, focusing on QMS implementation at their laboratories. A teach-back approach was used for the second week to develop knowledge sharing skills among the participants. The second workshop was conducted over one week, bringing together human and veterinary specialists for experience sharing. A local IQLS team composed of an international laboratory specialist and a national laboratory specialist provided direct on-site mentorship alone or in conjunction with visiting international experts, focusing on either veterinary or human health. IQLS teams worked in conjunction with the national quality focal persons on these visits. Onsite visit activities included review of previously developed action plans for furthering progress with laboratory management and quality-focused personnel. Each laboratory was encouraged to form a quality team that would meet weekly to deliberate on QMS activities. Further follow-ups utilized an adapted checklist from the World Health Organization's (WHO) Laboratory Quality Stepwise Improvement process (LQSI)[13], customized by IQLS into a user-friendly Microsoft Excel workbook and validated in other countries. This tool is based on the ISO 15189:2012 Medical laboratories — Requirements for quality and competence standard, broken down into four implementation phases, enabling laboratories to move towards accreditation in a step-by-step manner. Those phases are:
- Phase 1: Ensure that the primary process of the laboratory operates correctly and safely.
- Phase 2: Control and assure quality in a traceable fashion.
- Phase 3: Ensure proper management, leadership, and organization.
- Phase 4: Create continuous improvement and prepare for accreditation.
This approach was chosen by the NCDCP top management, as their Central facility laboratory was targeting accreditation for several analytes. A practical tool developed by IQLS to assess veterinary laboratories (animal health and/or food safety) previously used in Mali and Mauritania (2016) and Pakistan (2017–2018) was selected for the animal sector and customized for Armenia. These tools assisted facilities with tracking implementation of activities. Additional activities included supporting facilities with development and review of standard operating procedures (SOPs) and guidelines, as well as their adaptation and adoption at lower-level facilities.
Assessment process, data capture, and analysis
References
- ↑ 1.0 1.1 Wilson, Michael L; Fleming, Kenneth A; Kuti, Modupe A; Looi, Lai Meng; Lago, Nestor; Ru, Kun (1 May 2018). "Access to pathology and laboratory medicine services: a crucial gap" (in en). The Lancet 391 (10133): 1927–1938. doi:10.1016/S0140-6736(18)30458-6. https://linkinghub.elsevier.com/retrieve/pii/S0140673618304586.
- ↑ Balajee, S. Arunmozhi; Arthur, Ray; Mounts, Anthony W. (1 December 2016). "Global Health Security: Building Capacities for Early Event Detection, Epidemiologic Workforce, and Laboratory Response" (in en). Health Security 14 (6): 424–432. doi:10.1089/hs.2015.0062. ISSN 2326-5094. http://www.liebertpub.com/doi/10.1089/hs.2015.0062.
- ↑ 3.0 3.1 Global Health Security Agenda. "Laboratory Systems". Global Health Security Agenda. https://ghsagenda.org/laboratory-systems/. Retrieved 01 December 2020.
- ↑ World Health Organization, ed. (2016). International health regulations (2005) (Third edition ed.). Geneva, Switzerland: World Health Organization. ISBN 978-92-4-158049-6.
- ↑ Dzau, Victor; Fuster, Valentin; Frazer, Jendayi; Snair, Megan (28 September 2017). "Investing in Global Health for Our Future" (in en). New England Journal of Medicine 377 (13): 1292–1296. doi:10.1056/NEJMsr1707974. ISSN 0028-4793. http://www.nejm.org/doi/10.1056/NEJMsr1707974.
- ↑ Flatland, Bente (1 June 2012). "Veterinary laboratory quality management - it takes a village" (in en). Veterinary Clinical Pathology 41 (2): 171–173. doi:10.1111/j.1939-165X.2012.00442.x. https://onlinelibrary.wiley.com/doi/10.1111/j.1939-165X.2012.00442.x.
- ↑ World Health Organization (April 2008). "Joint WHO – CDC Conference on Health Laboratory Quality Systems" (PDF). World Health Organization. https://www.who.int/csr/ihr/lyon/report20080409.pdf.
- ↑ Yang, Ji-Rong; Teng, Hwa-Jen; Liu, Ming-Tsan; Li, Shu-Ying (1 April 2017). "Taiwan's Public Health National Laboratory System: Success in Influenza Diagnosis and Surveillance" (in en). Health Security 15 (2): 154–164. doi:10.1089/hs.2016.0104. ISSN 2326-5094. PMC PMC5404250. PMID 28418742. http://www.liebertpub.com/doi/10.1089/hs.2016.0104.
- ↑ Fitzmaurice, Arthur G.; Mahar, Michael; Moriarty, Leah F.; Bartee, Maureen; Hirai, Mitsuaki; Li, Wenshu; Gerber, A. Russell; Tappero, Jordan W. et al. (1 December 2017). "Contributions of the US Centers for Disease Control and Prevention in Implementing the Global Health Security Agenda in 17 Partner Countries". Emerging Infectious Diseases 23 (13). doi:10.3201/eid2313.170898. ISSN 1080-6040. PMC PMC5711326. PMID 29155676. http://wwwnc.cdc.gov/eid/article/23/13/17-0898_article.htm.
- ↑ Zheng, Zhe; Lu, Yi; Short, Kirsty R.; Lu, Jiahai (1 December 2019). "One health insights to prevent the next HxNy viral outbreak: learning from the epidemiology of H7N9" (in en). BMC Infectious Diseases 19 (1): 138. doi:10.1186/s12879-019-3752-6. ISSN 1471-2334. PMC PMC6371560. PMID 30744562. https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-019-3752-6.
- ↑ "One Health". World Health Organization. 21 September 2017. https://www.who.int/news-room/questions-and-answers/item/one-health.
- ↑ Centers for Disease Control and Prevention. "HHS and USDA Select Agents and Toxins - 7CFR Part 331, 9 CFR Part 121, and 42 CFR Part 73". Federal Select Agent Program. Centers for Disease Control and Prevention. https://www.selectagents.gov/sat/list.htm. Retrieved 17 June 2020.
- ↑ World Health Organization. "Laboratory Quality Stepwise Implementation tool". World Health Organization. https://extranet.who.int/lqsi/content/homepage. Retrieved 01 December 2020.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation, spelling, and grammar. We also added PMCID and DOI when they were missing from the original reference. A citation related to One Health (WHO 2017) was added to the introduction as the concept of what One Health is is never formally introduced. No other modifications were made in accordance with the "no derivatives" portion of the distribution license.