Difference between revisions of "Template:Article of the week"
Shawndouglas (talk | contribs) |
Shawndouglas (talk | contribs) (Updated article of the week text.) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File: | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Udesky EnviroHealth2019 18.png|240px]]</div> | ||
'''"[[Journal: | '''"[[Journal:Wrangling environmental exposure data: Guidance for getting the best information from your laboratory measurements|Wrangling environmental exposure data: Guidance for getting the best information from your laboratory measurements]]"''' | ||
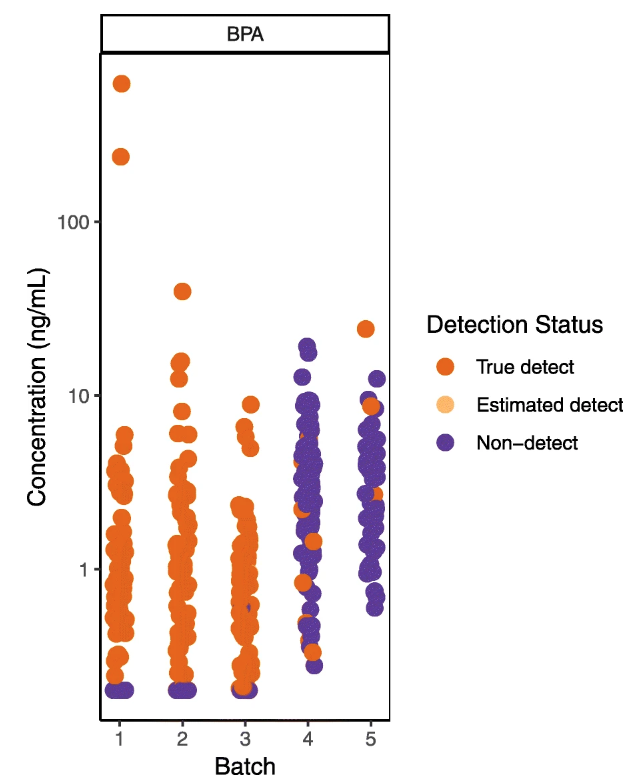
[[Environmental health]] and exposure researchers can improve the quality and interpretation of their chemical measurement data, avoid spurious results, and improve analytical protocols for new chemicals by closely examining lab and field [[quality control]] (QC) data. Reporting QC data along with chemical measurements in biological and environmental [[Sample (material)|samples]] allows readers to evaluate data quality and appropriate uses of the data (e.g., for comparison to other exposure studies, association with health outcomes, use in regulatory decision-making). However many studies do not adequately describe or interpret QC assessments in publications, leaving readers uncertain about the level of confidence in the reported data. One potential barrier to both QC implementation and reporting is that guidance on how to integrate and interpret QC assessments is often fragmented and difficult to find, with no centralized repository or summary. In addition, existing documents are typically written for regulatory scientists rather than environmental health researchers, who may have little or no experience in analytical chemistry. ('''[[Journal:Wrangling environmental exposure data: Guidance for getting the best information from your laboratory measurements|Full article...]]''')<br /> | |||
<br /> | <br /> | ||
''Recently featured'': | ''Recently featured'': | ||
Revision as of 17:55, 29 April 2021
Environmental health and exposure researchers can improve the quality and interpretation of their chemical measurement data, avoid spurious results, and improve analytical protocols for new chemicals by closely examining lab and field quality control (QC) data. Reporting QC data along with chemical measurements in biological and environmental samples allows readers to evaluate data quality and appropriate uses of the data (e.g., for comparison to other exposure studies, association with health outcomes, use in regulatory decision-making). However many studies do not adequately describe or interpret QC assessments in publications, leaving readers uncertain about the level of confidence in the reported data. One potential barrier to both QC implementation and reporting is that guidance on how to integrate and interpret QC assessments is often fragmented and difficult to find, with no centralized repository or summary. In addition, existing documents are typically written for regulatory scientists rather than environmental health researchers, who may have little or no experience in analytical chemistry. (Full article...)
Recently featured: