Journal:Institutional ELN-LIMS deployment: Highly customizable ELN-LIMS platform as a cornerstone of digital transformation for life sciences research institutes
| Full article title |
Institutional ELN-LIMS deployment: Highly customizable ELN-LIMS platform as a cornerstone of digital transformation for life sciences research institutes |
|---|---|
| Journal | EMBO Reports |
| Author(s) | Argento, Nicolas |
| Author affiliation(s) | École Polytechnique Fédérale de Lausanne |
| Year published | 2020 |
| Volume and issue | 21(3) |
| Article # | e49862 |
| DOI | 10.15252/embr.201949862 |
| ISSN | 1469-3178 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.embopress.org/doi/full/10.15252/embr.201949862 |
| Download | https://www.embopress.org/doi/epdf/10.15252/embr.201949862 |
Abstract
The systematic recording and management of experimental data in academic life science research remains an open problem. École Polytechnique Fédérale de Lausanne (EPFL) engaged in a program of deploying both an electronic laboratory notebook (ELN) and a laboratory information management system (LIMS) six years ago, encountering a host of fundamental questions at the institutional level and within each laboratory. Here, based on our experience, we aim to share with research institute managers, principal investigators (PIs), and any scientists involved in a combined ELN-LIMS deployment helpful tips and tools, with a focus on surrounding yourself with the right people and the right software at the right time. In this article we describe the resources used, the challenges encountered, key success factors, and the results obtained at each phase of our project. Finally, we discuss the current and next challenges we face, as well as how our experience leads us to support the creation of a new position in the research group: the laboratory data manager.
Keywords: electronic laboratory notebook, laboratory information management system, life sciences, research, laboratory data management
Introduction
Research tools in the life sciences are continuously evolving and improving, and scientists have always been eager to use the latest equipment. Ironically though, their main method of recording and managing experimental data has remained largely the same for centuries, with the paper-based laboratory notebook still the main method of record‐keeping. (Fig. 1) The adoption of electronic laboratory notebooks (ELNs) in academic laboratories has been slow, if laboratories have actually shown any interest at all. Their implementation necessitates institutional support[1], and despite much discussion of ELNs in the literature[2][3], success stories and recipes for their deployment remain scarce.[4][5][6] Moreover, although ELNs can improve efficiency in data capturing and reuse, they lack the features to rigorously document data critical for experimental reproducibility, such as sample traceability data and standard operating procedures (SOP). These features are, however, part of another tool for data management called the laboratory information management system (LIMS). (See Table 1 for a comparison of the two.)
|
| ||||||||
In order to encourage adoption of ELNs at the institutional level, École Polytechnique Fédérale de Lausanne (EPFL) started a dedicated program for ELN-LIMS deployment six years ago that involved research institute managers, principal investigators (PIs), and scientists at all levels. Here, we share the challenges, key success factors, and the results obtained at each phase of our project. Afterwards, we discuss the current and upcoming challenges and how our experience led us to support the creation of a new position in research groups: the laboratory data manager.
The project
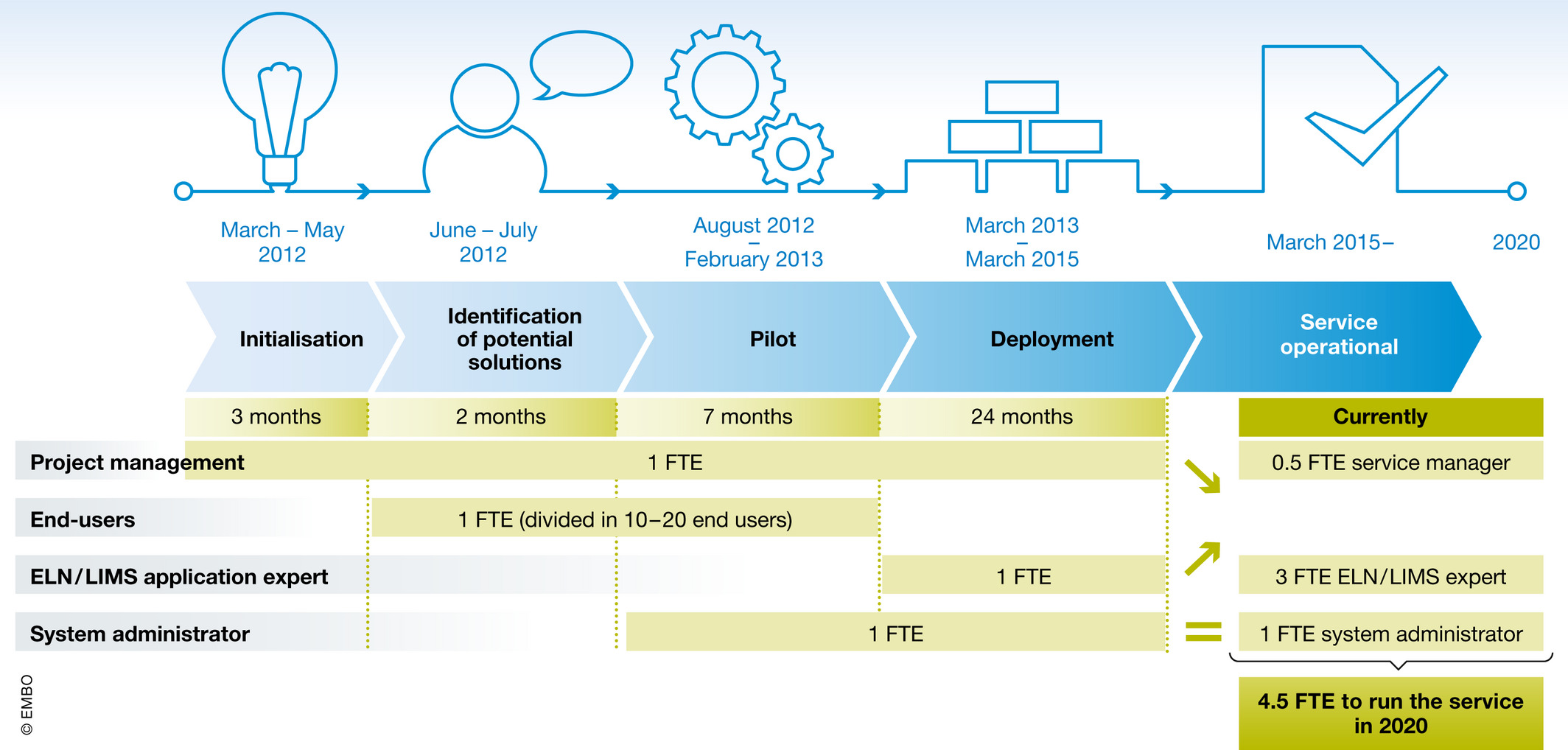
Our efforts towards ELN-LIMS deployment began in March 2012 and has run through five distinct phases, as shown in Fig. 2.
|
In the following sections, we present the step‐by‐step approach to deploy the platform in a laboratory. From our experience, each step contributed to successful and sustained ELN-LIMS adoption and use.
Initiating the project
By definition, an institutional ELN and LIMS project involves management, PIs, scientists at all levels, and the institute's IT department. As such, the steering committee for our project therefore included the Vice President of Research, who is responsible for scientific information, and the Vice President of Information Systems, who is responsible for IT governance and IT core services on campus. Representing the main users, the Life Sciences Faculty's Dean chaired the committee. This executive body defined the overall goals: to rationalize laboratories’ efforts, reduce waste of time and money, reduce loss and enable reuse of data, improve the reproducibility of experiments, and facilitate data sharing for collaborative projects.
The steering committee hired a project manager to coordinate the interests and requirements of the multiple stakeholders from distant fields, which made this project as complex as it was fascinating. To ensure the introduction of an ELN-LIMS service was suitable for those affected, we involved a panel of scientists. The project manager also played the role of a “business analyst” by meeting and surveying 25 laboratories whose needs and demands were synthesized in a weighted wish list that, along with legal requirements, helped to choose the ELN-LIMS solution. For instance, our institutional rules and laws about privacy and the use of human data prevented us from using a cloud‐based solution.
Identification of a suitable ELN-LIMS platform
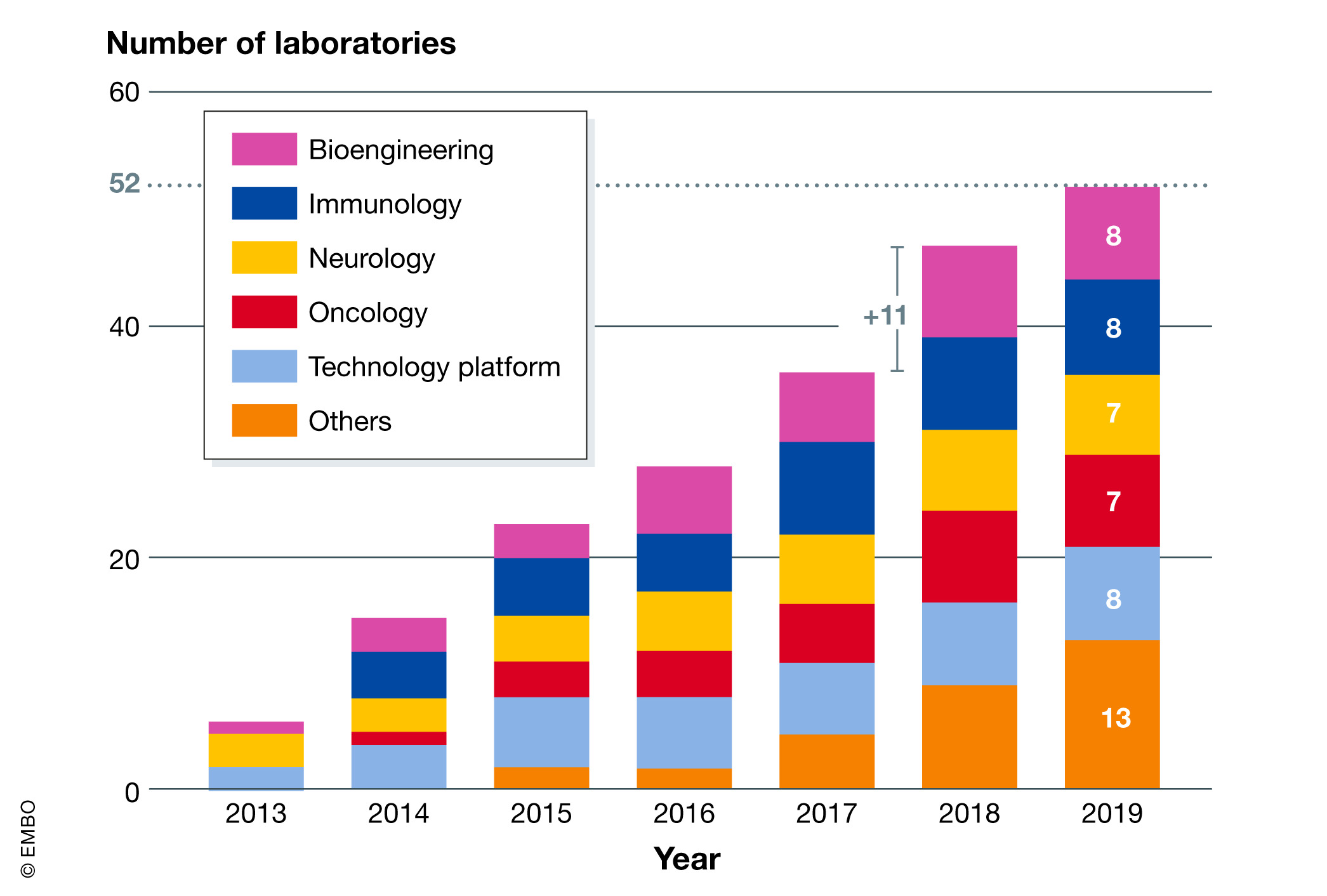
The initiation phase revealed that the project required more than just picking a commercial off‐the‐shelf (COTS) ELN. Our key users were active in bioengineering, immunology, neurology, and oncology, with correspondingly diverse approaches and demands, though developers of technology platforms also showed a strong interest (Fig. 3). Those stakeholders' routine workflows required additional functionality and administrative support. An analysis of those needs highlighted difficulties in managing data related to laboratory SOPs and samples, which are crucial for data reusability and experimental reproducibility in biomedical research.[7] Samples and experiments with human data require more sophisticated privacy management, a requirement that is increasing with the rise of personalized medicine. Feedback from laboratory staff also indicated that integration with third‐party information systems would be of added value for everyday work. For example, importing data from an animal facility was relevant to the experimental laboratories, while integration with work request forms and integration with the billing system were key features for technology platforms. The issue of authentication was also raised by laboratories and IT staff, for ease of use for the first group and security for the second group.
|
Involving the IT department from the beginning prevented us from taking obvious wrong directions in terms of technical choice. A striking example of their input was highlighting the heterogeneity of the scientists’ personal computers in the research institute, which made the choice of a web‐based solution almost mandatory, rather than installing software on each computer. However, the most interesting technical question was how to manage the diversity of needs. And the most important question for scientists was how to preserve creativity and freedom of research without introducing new technological burdens and hassles.
It was important that individual laboratories using our services remain architects of their own information system so as to preserve and maintain freedom and creativity. An ELN typically has highly standardized features, but new software technologies allow the creation of highly customizable databases and graphical user interfaces. The software we chose uses visual, declarative techniques instead of programming to enable fast, iterative, collaborative, and tailored implementation. Applications can be rapidly modified and maintained centrally.
Mastering such a powerful toolbox required the appointment of skilled people and best practices of implementation. This investment was counterbalanced by the possibility of the inclusion of a wide range of data, development of home-made features (e.g., financial management, work request forms), and integration with other information systems. It also opened the possibility for compliance with ISO 9001 or 21 CFR Part 11 standards that are required by some technology platforms. Those standards' quality assurance and data privacy concerns affect many industries and can also help to foster reproducibility in the life sciences.[8][9]
Pilot and full deployment
To accurately assess the personnel and skills needed to install and configure the platform, and then train the staff, a dedicated budget to run a pilot phase was required. This budget covered a six‐month license fee for the ELN-LIMS platform and a system administrator along with training and support. Through this pilot, five volunteer laboratories began to configure and use the ELN-LIMS platform. At the end, all stakeholders validated the choice of the solution over the short and long term, and the steering committee approved deployment at a larger scale.
The same staff then organized the full deployment. A dedicated ELN-LIMS platform engineer was hired to help laboratories to get the most from the customizable platform (Fig 2). Such an ELN-LIMS expert needed to have strong IT competencies, project management skills, and good general knowledge in research to efficiently communicate with the scientific staff. More specifically, the engineer developed and optimized the work methodology to ensure sustainable growth from a technical and scientific point of view. The announcement of the Swiss National Science Foundation (SNSF) to make a data management plan mandatory for all grant applications from October 2017[10] created a peak of demand for our services in 2018, when 10 laboratories voluntarily started ELN-LIMS deployment (Fig. 3). Nonetheless, laboratories were not forced to use the ELN-LIMS, and it still remains a PI's decision to use the platform. Fig. 4 shows examples of the current typical usage of the ELN-LIMS platform.
|
Each ELN-LIMS platform deployment is managed as a separate sub‐project, since the information systems are tailor‐made for each laboratory. The deployment phase starts with informal discussions between the ELN-LIMS application expert and the laboratory's management to demonstrate the offered services. This introduction aims to confirm that the tool could support the laboratory's objectives. If so, the list of objectives is formalized and validated by the project sponsor, the PI in this case. Objectives are prioritized (Box 1) and have an appointed reference person. Roles and responsibilities must be clarified.
|
The stakeholders and their roles in the deployment
The PI is the sponsor, who initiates the project and assumes overall responsibility. He or she usually delegates the work to appropriate staff members. Along the deployment, the PI can be asked to make decisions on proposals.
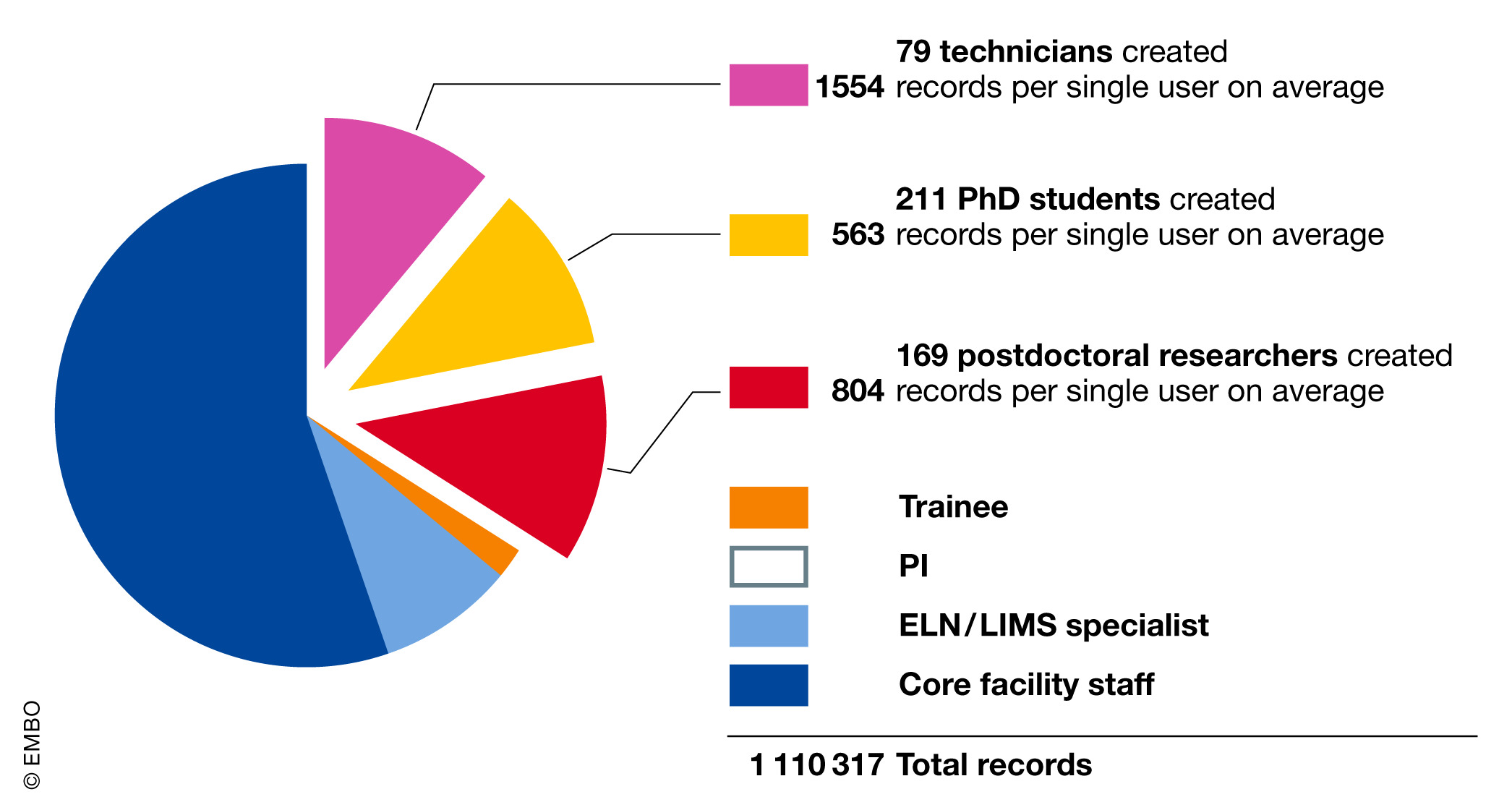
Laboratory referents take the lead and responsibility to fulfill the aims delegated by the PI. Initially, this task was given to newly arrived PhD students, but we soon realized that a thorough knowledge of the laboratory operation is required for efficient implementation. This role should therefore be given to experienced scientists or technicians; the latter often have associated laboratory planning tasks and are less likely to leave than scientific staff. Those considerations make them more prone to act as a catalyst in adopting the ELN-LIMS platform. This view is supported by raw record creation quantity as a function of staff position (Fig. 5).
|
The ELN-LIMS application expert is responsible for configuring the system according to the laboratory referents’ requests and to advise about best practices; they translate the identified needs into configuration and code. A strong know‐how in ELN-LIMS customization, best practices, and general knowledge about the scientific field are tremendously important. By working in several laboratories, our ELN-LIMS expert team developed strong skills in laboratory data management and project management that the typical laboratory does not have. Consequently, they currently work as a deployment project manager, whereas ideally, their role should be restricted to supporting the workflows of the adopting laboratory, not imposing (well‐intentioned) ideas from the outside.
ELN-LIMS end users are the laboratory staff and PIs, and their active participation in the deployment and their remarks and comments are crucial for setting up a tool that fits their needs and habits. While sample and SOP management are usually easily adopted, the use of the ELN is trickier as it offers a large range of possibilities to organize projects and experiments, compared to paper notebooks. This can make adaptation frustrating and can take weeks; indeed, some laboratories decided not to use the ELN or only a part of the staff adopted it. We have also seen a few laboratories abandon the ELN. Close support during the first week of usage and regular communication are necessary to reduce the impact of initial difficulty of use. Here, the normal turnover of scientific staff in research laboratories can be used as an opportunity. It is problematic to ask a post‐doctoral researcher or a PhD student to change their data management tools and habits in the middle of their project, whereas new recruits can start fresh with the ELN-LIMS system.
Configuration and go-live
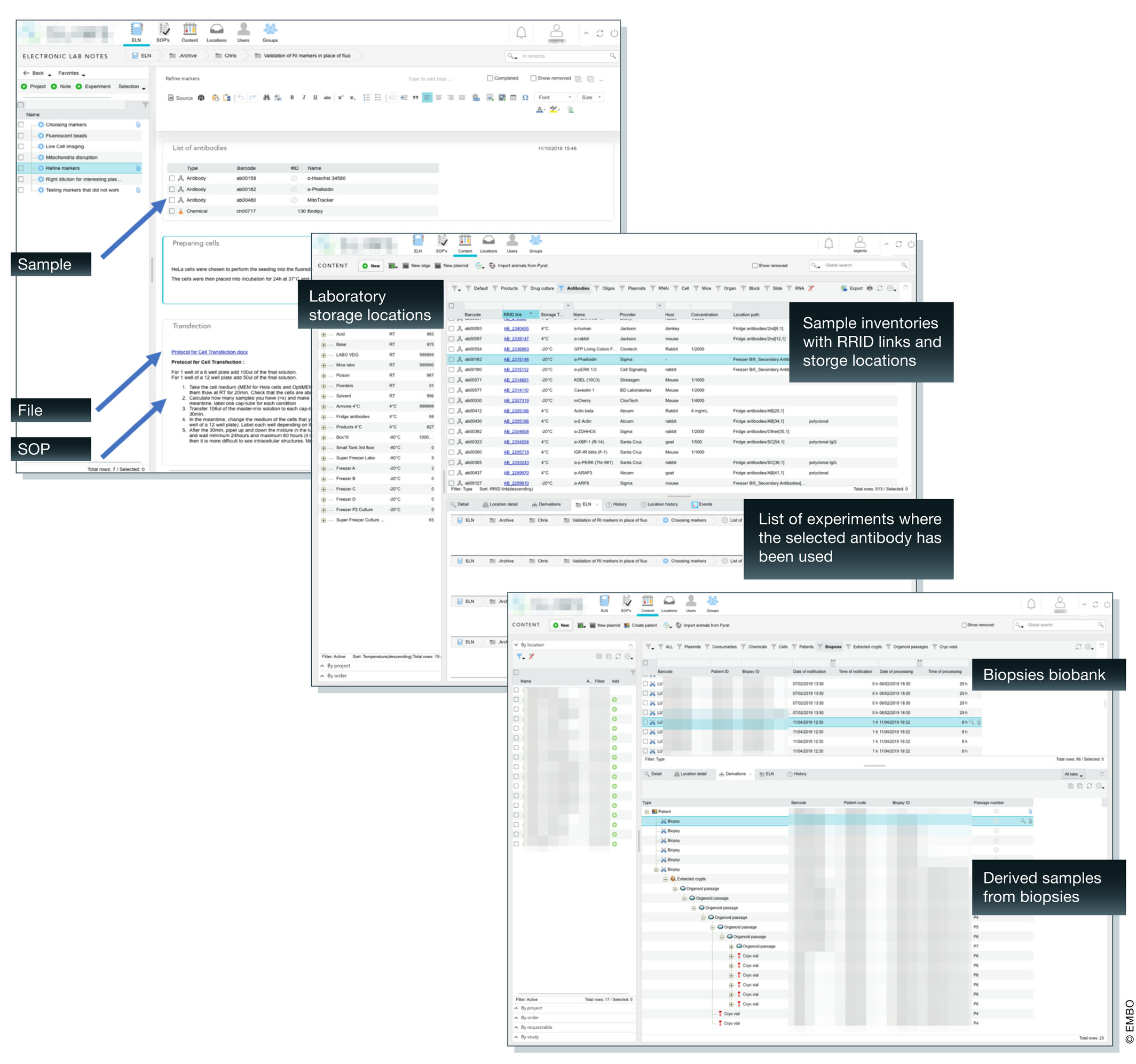
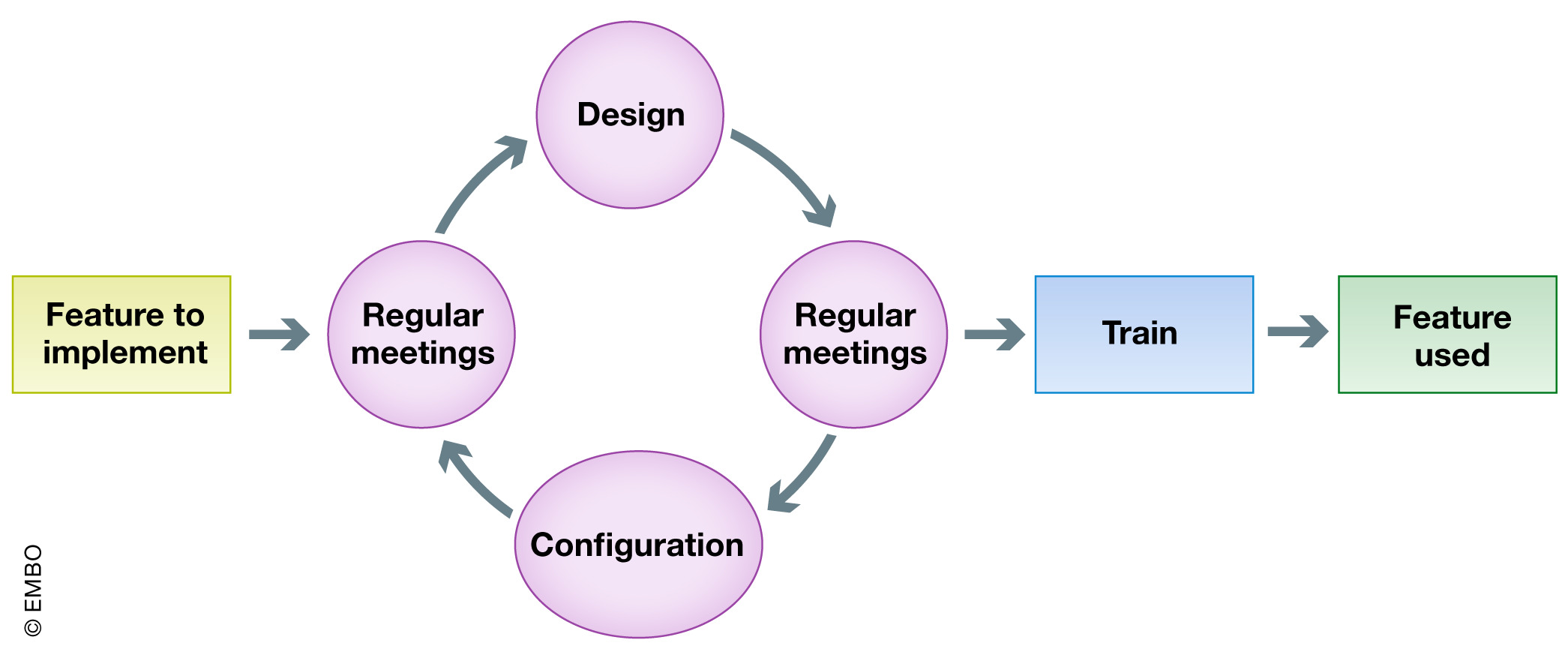
In this phase, the ELN-LIMS expert needs to work as closely as possible with researchers to translate laboratory context, culture, and workflow into the configuration. According to our experience, the conception must be an incremental process. This process is common practice in the software industry's “agile” methodology, one based on continuous small deliveries and short daily meetings.[11] While preparing for deployment of the system (or additional components), we organize regular meetings to discuss the ELN-LIMS platform configuration and design and, depending on the test performed by the referent, further adapt the configuration for the next meeting (Fig. 6). The cycle is repeated until the result is sufficiently convincing to validate the deployment of this feature and corresponding training.
|
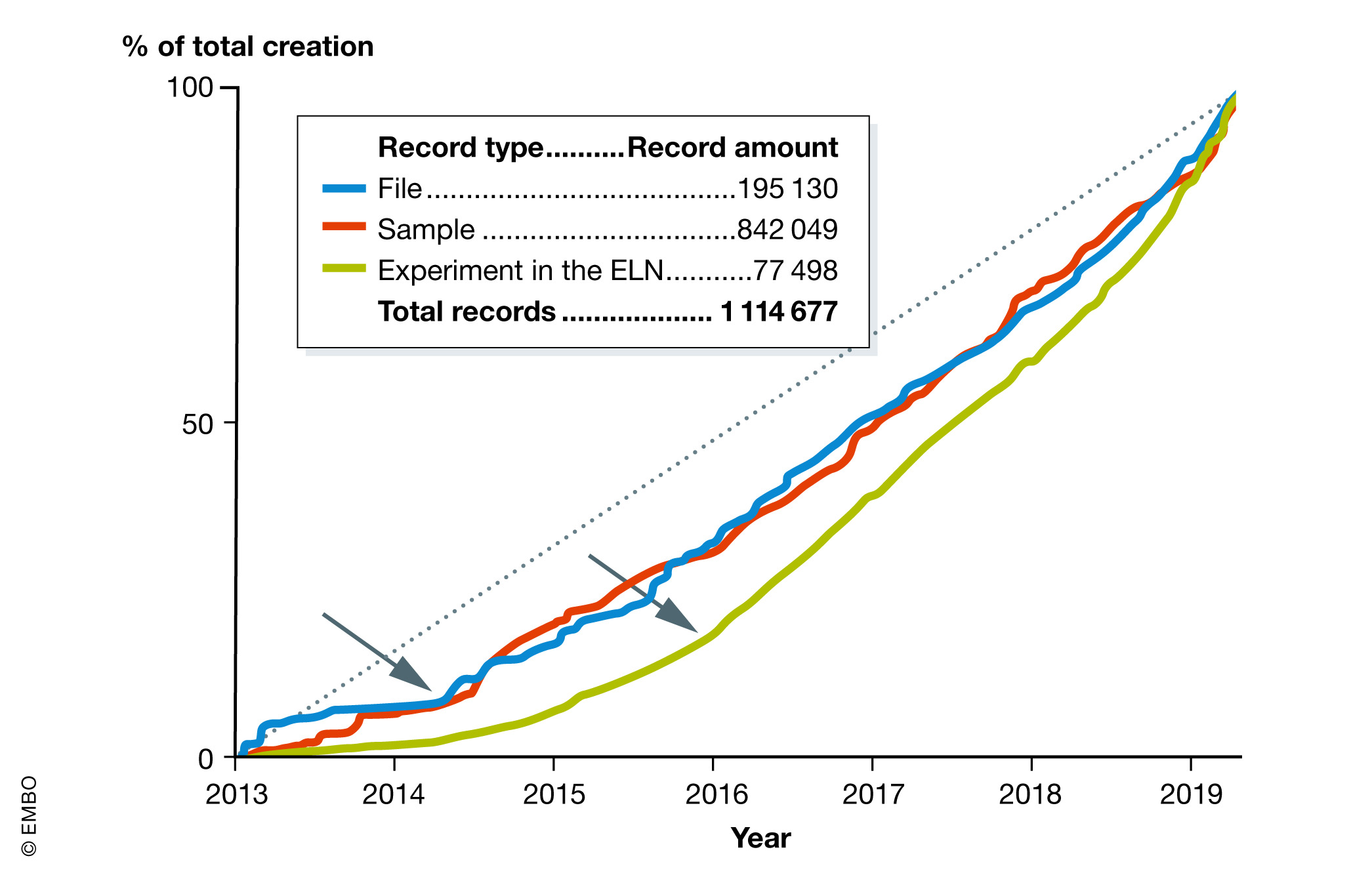
For almost five years, we have been serving the original laboratories involved with the project as well as new volunteer laboratories. The raw record creation (files and samples) in the platform accelerated in mid‐2014 when technology platforms joined the system (Fig. 7). The number of created experiments in the ELN (green) has grown more rapidly since 2016, reflecting improvement in training, communication between stakeholders, and continuous upgrades.
|
Although the service is hosted at the School of Life Sciences IT department, the platform has been requested and used by biologists from other faculties on the campus (Fig 3). As of early 2020, three full‐time ELN-LIMS application expert staff serve 52 laboratories. This team is composed of people with heterogenous competencies from bioengineering and chemistry to computer sciences and IT management. In addition, a full-time system administrator operates the servers and data storage, while a part-time assistant ensures the service management. The whole service requires four full-time employees and one part-time employee (Fig 2) to guarantee the system's sustainability and evolution. They ensure day‐to‐day assistance with user deployments and training sessions while coordinating and performing the regular maintenance of the infrastructure. A frequently overlooked, yet important and time‐consuming task they manage is to ensure platform upgrades, which has a key impact on user experiences and data integrity.
One of our ELN-LIMS configurations is used by an “industrial” technology platform that sterilizes glass, decontaminates waste, and prepares standard solutions. They are ISO 9001-certified, and their data management is audited every year. As we operate the major part of their information systems, we are audited too, and thus we have also put a quality system in place for maintenance and data backup. Generally, technology platforms are natural customers of such services. They run more standardized experiments with higher requirements for traceability. (Figure 5 reminds us that they are the main record producer in the ELN-LIMS platform.)
The laboratory data manager
We experienced that ELN-LIMS application experts were regularly asked by laboratory referents to organize their data management. However, data management should be driven by science and not vice versa, which is one of the reasons we propose a “laboratory data manager” for managing sensitive or crucial data.
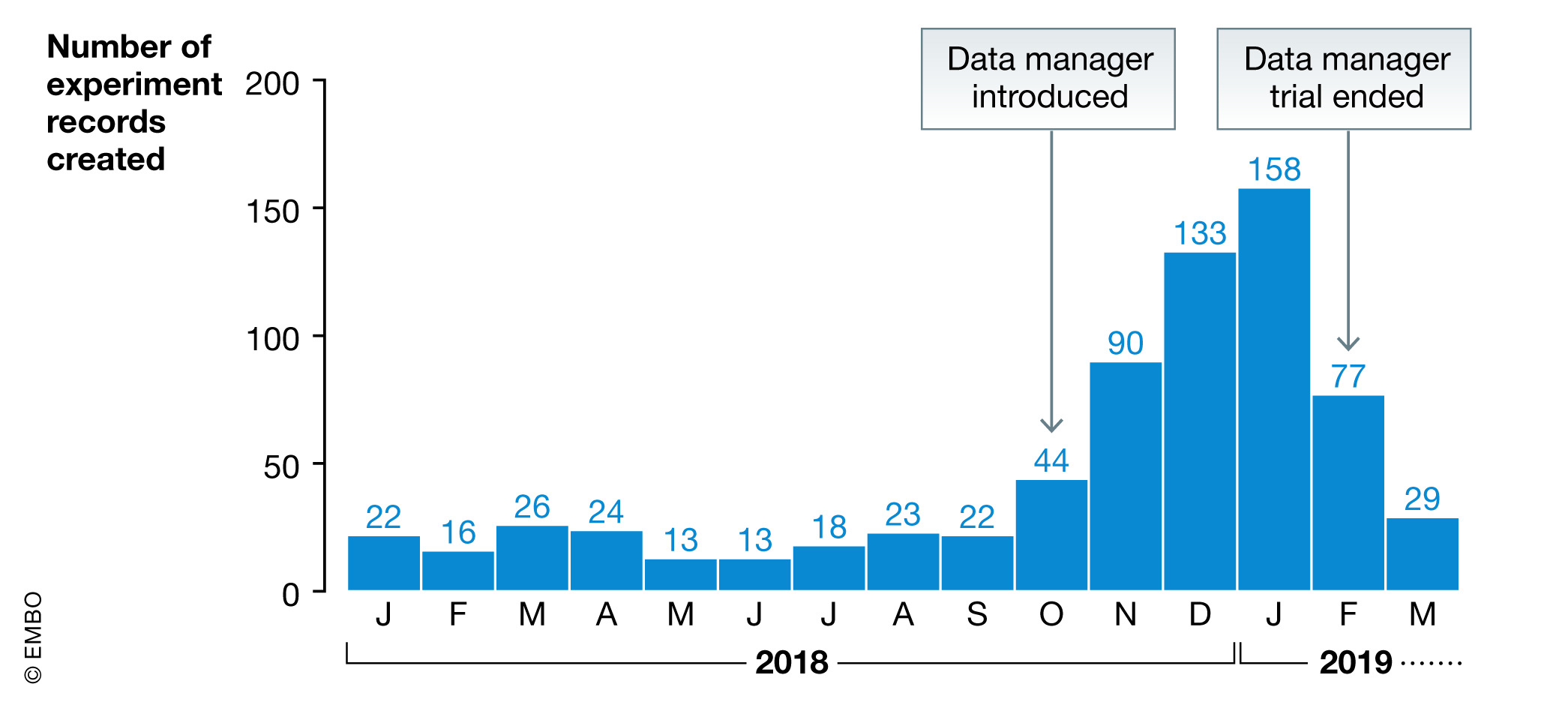
Obviously, the particular skills required for a data manager in cutting‐edge research is a challenge. As a trial, we placed a properly skilled data manager into one of our research groups for a couple of months. As shown in Fig. 8, it had an immediate impact on the amount of data collected in the ELN and enabled the laboratory to review their data management practices. We do not have information about the quality of the data produced, but we expect improvement at this level, and we anticipate that the publishing process could also be accelerated, owing to better reusability of data inside the laboratory.
In all, a laboratory data manager would apply general institutional or research‐specific policies and good practices, and convert general infrastructure into daily practical solutions that fit the localized needs of the laboratory. Being integrated with the laboratory is necessary to legitimize changing practices while maintaining the flexibility and freedom required by science. Note that a data manager would not necessarily be a full‐time job, depending on the laboratory's activity.
|
Conclusions
In summary, we believe our institutional project showed that the ELN-LIMS platform helps to capture and record data, and helps to make the data to become findable, accessible, interoperable, and reusable (FAIR). The reusability criterion implies quality improvements that are more dependent on data management practice than on the available electronic tools.
Several challenges for the success of data management yet remain. One challenge is the slow adoption of the ELN by research staff, partly because the ELN is still a work in progress, partly because old working habits are slow to change. Furthermore, managing huge files—e.g., genomic data or microscopy images—remains a major challenge, as is archiving the data collected by the ELN-LIMS systems. Finally, integration with other elements of the institutional information system is technically possible, but requires governance of business applications, interface development, and resources for maintenance. Amidst all of these challenges, we strongly believe funding the position of data managers remains a priority. In the end, it is the daily practice of the scientists that will drive and sustain the digital transformation of the life sciences.
Acknowledgements
I acknowledge Gaël Anex and all the School of Life Sciences and EPFL directors for creating this favorable environment, improving laboratory data management; Pr. Andy Oates and Pierig Le Pottier for their strong support and help in writing the article; my colleagues from the IT department, in particular Paul Schalbetter, for the scripting of the figures, as well as Philippe Borel, Peter Hliva, and Christopher Tremblay who ensure the continuity of ELN-LIMS deployments; and finally all the scientific staff who actively fostered and participated in the ELN and LIMS deployments in their laboratory.
Funding
The EPFL, School of Life Sciences provided funding for this research.
References
- ↑ Ash, J.S.; Anderson, N.R.; Tarczy-Hornoch, P. (2008). "People and Organizational Issues in Research Systems Implementation". JAMIA 15 (3): 283–9. doi:10.1197/jamia.M2582. PMC PMC2410012. PMID 18308986. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2410012.
- ↑ Kwok, R. (2028). "How to Pick an Electronic Laboratory Notebook". Nature 560 (7717): 269-270. doi:10.1038/d41586-018-05895-3. PMID 30082695.
- ↑ Menzel, J.; Weil, P.; Bittihn, P. et al. (2013). "Requirement analysis for an electronic laboratory notebook for sustainable data management in biomedical research". Studies in Health Technologies and Informatics 192: 1108. doi:10.3233/978-1-61499-289-9-1108. PMID 23920882.
- ↑ Guerrero, S.; Dujardin, G.; Cabrera-Andrade, A. et al. (2016). "Analysis and Implementation of an Electronic Laboratory Notebook in a Biomedical Research Institute". PLoS One 11 (8): e0160428. doi:10.1371/journal.pone.0160428. PMC PMC4968837. PMID 27479083. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4968837.
- ↑ Dirnagl, U.; Przesdzing, I. (2016). "A pocket guide to electronic laboratory notebooks in the academic life sciences". F1000Research 5: 2. doi:10.12688/f1000research.7628.1. PMC PMC4722687. PMID 26835004. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4722687.
- ↑ Riley, E.M.; Hattaway, H.Z.; Felse, P.A. (2017). "Implementation and use of cloud-based electronic lab notebook in a bioprocess engineering teaching laboratory". Journal of Biological Engineering 11: 40. doi:10.1186/s13036-017-0083-2. PMC PMC5701295. PMID 29201138. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5701295.
- ↑ Freedman, L.P.; Cockburn, I.M.; Simcoe, T.S. (2015). "The Economics of Reproducibility in Preclinical Research". PLoS Biology 13 (6): e1002165. doi:10.1371/journal.pbio.1002165. PMC PMC4461318. PMID 26057340. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4461318.
- ↑ Dirngal, U.; Kurreck, C.; Castaños-Vélez, E. et al. (2018). "Quality Management for Academic Laboratories: Burden or Boon? Professional Quality Management Could Be Very Beneficial for Academic Research but Needs to Overcome Specific Caveats". EMBO Reports 19 (11): e47143. doi:10.15252/embr.201847143. PMC PMC6216282. PMID 30341068. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6216282.
- ↑ Riedl, D.H.; Dunn, M.K. (2013). "Quality Assurance Mechanisms for the Unregulated Research Environment". Trends in Biotechnology 31 (10): 552-4. doi:10.1016/j.tibtech.2013.06.007. PMID 24054820.
- ↑ Swiss National Science Foundation (6 March 2017). "Open Research Data: Data management plans will be introduced in project funding". http://www.snf.ch/en/researchinFocus/newsroom/Pages/news-170306-towards-open-research-data.aspx.
- ↑ Conforto, E.C.; Salum, F.; Amaral, D.C. et al. (2014). "Can Agile Project Management Be Adopted by Industries Other than Software Development?". Project Management Journal 45 (3): 21–34. doi:10.1002/pmj.21410.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Grammar was cleaned up for smoother reading. In some cases important information was missing from the references, and that information was added. The inline link to the SNSF article was turned into a formal citation for this version. Additional section headers were made to make the material flow better.