Journal:Methods for quantification of cannabinoids: A narrative review
| Full article title | Methods for quantification of cannabinoids: A narrative review |
|---|---|
| Journal | Journal of Cannabis Research |
| Author(s) | Lazarjani, Masoumeh P.; Torres, Stephanie; Hooker, Thom; Fowlie, Chris; Young, Owen; Seyfoddin, Ali |
| Author affiliation(s) | Auckland University of Technology, Chapman University, ZeaCann Limited, |
| Primary contact | Email: Online form |
| Year published | 2020 |
| Volume and issue | 2 |
| Article # | 35 |
| DOI | 10.1186/s42238-020-00040-2 |
| ISSN | 2522-5782 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://jcannabisresearch.biomedcentral.com/articles/10.1186/s42238-020-00040-2 |
| Download | https://jcannabisresearch.biomedcentral.com/track/pdf/10.1186/s42238-020-00040-2.pdf (PDF) |
Abstract
Background: Around 144 cannabinoids have been identified in the Cannabis plant; among them tetrahydrocannabinol (THC) and cannabidiol (CBD) are the most prominent ones. Because of the legal restrictions on cannabis in many countries, it is difficult to obtain standards to use in research; nonetheless, it is important to develop a cannabinoid quantification technique, with practical pharmaceutical applications for quality control of future therapeutic cannabinoids.
Method: To find relevant articles for this narrative review paper, a combination of keywords such as "medicinal cannabis," "analytical," "quantification," and "cannabinoids" were searched for in PubMed, EMBASE, MEDLINE, Google Scholar, and Cochrane Library (Wiley) databases.
Results: The most common cannabinoid quantification techniques include gas chromatography (GC) and high-performance liquid chromatography (HPLC). Gas chromatography is often used in conjunction with mass spectrometry (MS) or flame ionization detection (FID). The major advantage of GC is with the quantification of terpenes. However, for evaluating acidic cannabinoids, it needs to be derivatized. The main advantage of HPLC is the ability to quantify both acidic and neutral forms of cannabinoids without derivatization, which is often accomplished with MS or ultraviolet (UV) detectors.
Conclusion: Based on the information presented in this review, the ideal cannabinoid quantification method is HPLC paired with tandem mass spectrometry (MS/MS).
Introduction
Cannabis sativa L. is an annual herbaceous flowering plant indigenous to eastern Asia.[1] The phenotypes of Cannabis are highly variable, and the plant is accepted to have two subspecies: C. sativa and C. indica.[2][3] A third variety, C. ruderalis, has been identified as a Cannabis species; however, it is not broadly recognized as a specific subspecies of C. sativa.[2][4] The Cannabis plant has been used for its therapeutic properties for thousands of years, and it was introduced to Western medicine in the nineteenth century, until it was later outlawed in the U.S. from the mid-1930s.[5]
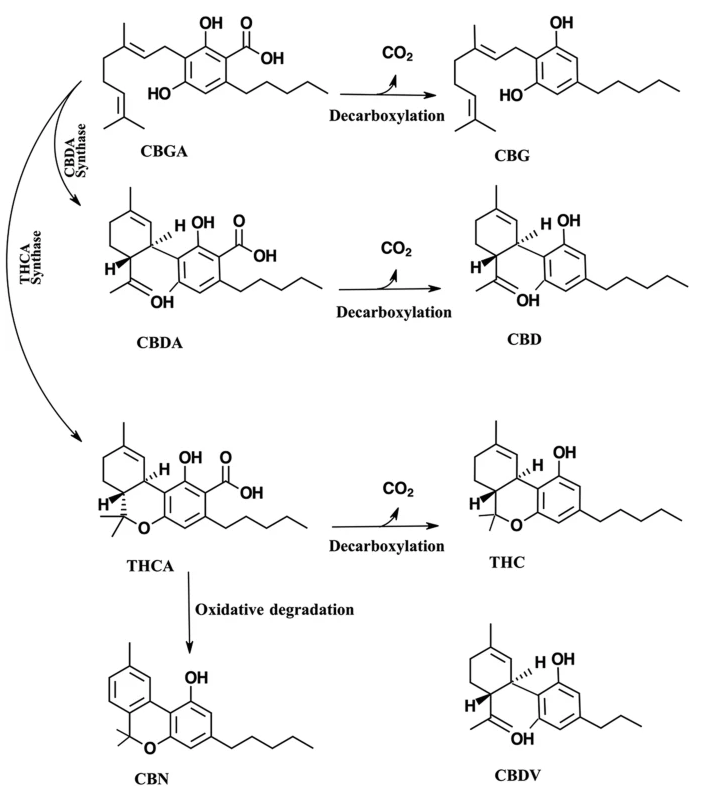
The medicinal compounds from Cannabis plants are mostly concentrated in the female flowers of this dioecious species.[4] The so-called resin is the source of a wide variety of terpenoids and cannabinoids.[4] The therapeutic properties of cannabis are attributed to cannabinoids.[6] Cannabinoids are found in the resin produced by the trichomes, which are widely distributed on both the male and female plants, though they are most highly concentrated on the female flowers of the cannabis plant.[1][7] Cannabinoids are terpenophenolic compounds unique to Cannabis.[2] To date, 144 cannabinoids have been identified.[6] The two cannabinoids most well known for their therapeutic properties are tetrahydrocannabinol (THC) and cannabidiol (CBD).[2][8] THC and CBD are the neutral homologs of tetrahydrocannabinolic acid (THCA) and cannabidiolic acid (CBDA), respectively.[8] A conventional classification model of cannabinoids is due to their chemical contents dividing them into eleven subclasses, including THC, CBD, cannabigerol (CBG), cannabichromene (CBC), cannabinol (CBN), (−)-Δ8-trans-tetrahydrocannabinol (Δ8-THC), cannabicyclol (CBL), cannabinodiol (CBND), cannabielsoin (CBE), cannabitriol (CBT), and "miscellaneous types."[9] (Fig. 1).
|
Because consumers have limited means to analyze the chemical composition of cannabis products, consumers may be inadvertently purchasing products with undesired properties given that different cannabinoids produce different effects.[11] As a result, it is important to implement methods of quality control so that consumers can be certain that what they are consuming will have the desired effects.[4][11][12] As cannabis use becomes progressively accepted, it becomes increasingly important to quantify the cannabinoid profile and content of cannabis preparations to ensure the uniformity and quality of the preparations.[13]
A variety of analytical techniques have been developed for the quantification and qualification of cannabinoids and other compounds in the Cannabis plant. Advances in analytical methods have also resulted in detection of various additional compounds from cannabis extracts in the last decade (e.g., terpenes). The purpose of this literature review is to explore cannabinoid quantification techniques and subsequently suggest an optimal method for pharmaceutical-grade quantification.
Methods
To find relevant papers for this narrative review, many databases were reviewed over a period of eight months. A combination of keywords such as "medicinal cannabis," "analytical," "quantification," and "cannabinoids" were searched. PubMed, EMBASE, MEDLINE, Google Scholar, and Cochrane Library (Wiley) databases were searched for English language papers published from 1967 to 2019. In the next step, the results were then scrutinized to discard irrelevant papers. Those papers which were deemed relevant were subjected to more detailed analysis. In total, the number of papers read was about 75, including around 15 irrelevant papers.
Quantitative analysis of cannabinoids
Gas chromatography
Gas chromatography (GC) is one of the most commonly used chromatographic methods in quantitative cannabinoid analysis.[14] Gas chromatography is typically completed in under 20 minutes at up to 300 °C and makes use of stationary phases with low polarities, such as 5% diphenyl- and 95% dimethylpolysiloxane.[15] It is important to note that the total quantity of cannabinoids in a sample is the sum of the acidic and neutral components.[7] Because gas chromatography requires high column temperatures, the acidic cannabinoids undergo decarboxylation during transit through the column.[1][7][14] As such, acidic cannabinoids cannot be determined unless they are derivatized prior to analysis.[14] Not only does derivatization preserve cannabinoid structure, but it also causes cannabinoids to become more volatile, thus improving peak shape.[15] Dussy et al.[12] suggested calculating the amount of neutral and acidic cannabinoids separately in order to accurately determine the total cannabinoid content. Gas chromatography resolves cannabinoids, but detection on elution presents its own challenges and solutions.
GC- FID/MS
GC is normally coupled with mass spectrometry (MS) or flame ionization detection (FID) to detect and quantify cannabinoids[7][14] (see Tables 1 and 2). MS employs standardized electron ionization to fragment analytes, permitting the use of compound libraries to identify the parent analyte. FID provides more accurate cannabinoid quantification because it makes use of relatively cheap authentic standards, whereas MS usually requires equivalent deuterated standards, which are expensive and not available for all cannabinoids.[7][14]
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
As shown in Table 1, all referenced instances used helium as the carrier gas in GC-MS, as it provides higher efficacy than other gases such as hydrogen and nitrogen. In each study, according to the compound of interest, different columns and different protocols were used. The high temperature in injection point is the con for preserving acidic forms of cannabinoids. To validate all the quantification methods, two parameters should be detected. Limit of detection (LOD) and limit of quantification (LOQ). These two parameters show the lowest concentration of the compound of interest that can be reliably measured by an analytical method (which are mentioned in Table 1 for the GC-MS method).
Leghissa et al.[22] used multiple reaction monitoring (MRM) analysis of cannabis from a surrogate hops matrix by GC-MS with triple quadrupole mass spectrometry for the first time. They used silylated cannabinoids to avoid the decarboxylation process due to high temperature typically found in the GC injection port. They found that this method is applicable to cannabinoid analysis from plant materials and cannabis products. The main achievement of their study is that, in this method, because the risk of interference from the essential oils and waxes is reduced, the extraction needs less sample preparation in the laboratory, compared to other techniques like solid-phase extraction (SPE).
In another study, GC with vacuum ultraviolet spectroscopy (GC-VUV) was used. The detection of cannabinoids and the cannabinoid metabolites was fast and simple, making it ideal for use in rapid detection of them even without having a baseline for cannabinoids for comparison. This method has just one disadvantage, which is a high LOD. Due to this drawback, detecting analytes in biological matrices cannot be accomplished without pretreatments.[23]
Two-dimensional gas chromatography
Experience has shown that one-dimensional gas chromatography does not provide enough resolution to analyze complex cannabinoid mixtures.[8] Two-dimensional gas chromatography (GC × GC) has been found to be preferable over one-dimensional GC for analyzing complex mixtures, such as cannabis extracts, in that it reveals more sample components.[13][24][25] Additionally, GC × GC produces two sets of retention data for sample constituents, which can greatly aid analyte identification.[24]
In the previous Table 2, nitrogen and helium are shown as the carrier gases. In many studies, it has been proved that nitrogen has the best efficacy, but it is time consuming. On the other hand, by using helium, the process is rapid and efficient, but the price is not affordable. The onitial and end temperatures, the type of columns, and the drawbacks are almost similar to GC-MS.
Liquid chromatography (LC)
High-performance liquid chromatography (HPLC) is a commonly used liquid chromatography (LC) technique in quantitative cannabinoid analysis[14] (Table 3). The most common columns used in HPLC consist of C18 stationary phases[7][15] and methanol with 0.1% formic acid or water with 0.1% formic acid as mobile phases.[15] C18 columns have high resolution and can differentiate between cannabinoids.[7][26] The use of formic acid in the mobile phase provides better peak shape and improved results, when compared with other mobile phases, as well as improved resolution in the chromatographic analysis.[26] In another example, Peschel and Politi[27] ran two HPLC assays to identify major and minor cannabinoids. Extract profiling was based on the main cannabinoid (THC, CBD, CBG, and CBN) quantification and the presence of acids and flavones. In this research, they found good resolutions of THCA, CBGA, CBDA, THCVA, THC, CBG, CBD, and THCV by HPLC.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
From Table 3, it is clearly obvious that C18 is the most popular column, as mentioned earlier. The main difference between HPLC and GC is the operating temperature. That is why HPLC is used when preserving the acidic form of cannabinoids matters. The only disadvantage of HPLC is its inability to analyze volatile compounds like terpenes.
HPLC-MS/UV/DAD
Different detection techniques can be used in conjunction with HPLC to analyze cannabinoids. Common detection methods include mass spectrometry (MS) and ultraviolet (UV) absorbance (190 to 400 nm)[5][15], with UV detection being much less expensive and more straightforward than MS detection.[15] Acidic cannabinoids show absorption peaks at around 270 nm and 310 nm, while neutral cannabinoids show absorption peaks at about 220 nm.[16][26] Citti et al.[26] developed a rapid HPLC technique with UV detection (HPLC-UV) to qualify and quantify major cannabinoids (CBDA, CBD, CBN, THC, and THCA) in cannabis extracts. However, absorption profiles from UV detection do not contain enough information to be used in isolation to accurately identify cannabinoids.[15] Much more information can be obtained by diode array detection (DAD), which covers the visible and UV spectrum. DAD can help to improve specificity because acidic and neutral cannabinoids have different absorption spectrums.[26][15] A use case can be found with Peschel and Politi[27], who used HPLC-DAD to differentiate between Cannabis sativa chemotypes, extracts of different polarity, and to profile extracts.
Nonetheless, all light absorbance detectors lack the specificity of MS[7][15], which is particularly useful in analyzing extracts from complex matrices such as cannabis. However, some cannabinoids, such as CBG and CBD are difficult to separate using UV detection, especially in concentrations greater than 10% in the extract.[1][7] In the case of CBG and CBD, MS is preferred because it can differentiate between different cannabinoids based on the m/z value of their molecular ion.[7] The m/z value is not always unique, however; in an ongoing study, Citti et al.[7] found five cannabinoids with the same m/z of 315.2294; this value matches that of THC and CBD in Bediol oil and ethanol extracts. Because some of these cannabinoids coelute, analysis of these compounds is difficult.
HPLC-ESI-qTOF/MS
HPLC-electrospray ionization-quadrupole-time-of-flight mass spectrometry (HPLC-ESI-qTOF/MS) is very effective in identifying complex and common compounds and can identify the main component of a sample, in addition to enhancing the signal-to-noise ratio in the peaks. Citti et al.[26] analyzed cannabinoid concentrations in olive oil, ethanol, and supercritical CO2 and found that UV-DAD and qTOF detectors produced similar results, thus suggesting that these two detection systems are equally useful in cannabinoid analysis. Pellati et al.[19] and Brighenti et al.[28] used HPLC-ESI/MS both in positive and negative ion mode for the analysis of cannabinoids. By developing HPLC methods, they improved resolution, peak shape, and separation performance, together with the improvement of the ionization in HPLC-ESI/MS.[19][28]
HPLC-MS/MS
A solution to analyzing coeluting cannabinoids is to use HPLC-tandem mass spectrometry (HPLC-MS/MS).[5][7] Aizpurua-Olaizola et al.[5] utilized HPLC-MS/MS to identify THCA, THC, CBD, THCV, CBG, and CBN in 30 Cannabis plant varieties. Using the results from their study, they were able to distinguish Cannabis plants grown indoors from those grown outdoors. These results suggest that HPLC can be used to successfully determine several cannabinoid profiles and that this method can be used to distinguish between cannabis varieties and growing conditions.[5]
LC-MS/MS with APCI
Grauwiler et al.[30] developed a method to simultaneously detect five cannabinoids in human plasma and urine using HPLC-MS/MS and atmospheric-pressure chemical ionization (APCI). Their method had a 25-minute runtime, with a 0.2 ng/mL lower limit of quantification on samples following human oral administration of 20 mg of THC. Although APCI methods are less sensitive than ESI methods, APCI methods were chosen instead of ESI methods because they produced fewer matrix effects. Limits of detection and limits of quantification were found to be acceptable even with APCI methods.[30]
UPLC-qTOF
Ultra-performance liquid chromatography allows researchers to use a thinner column compared to HPLC, and it can be used for particles less than 2 μm, which leads to better separation with higher speed than conventional HPLC. For example, Aizpurua-Olaizola et al.[5] identified seven unknown minor cannabinoids using UPLC-quadrupole-time-of-flight mass spectrometry (UPLC-qTOF). Jung et al.[31] also implemented qTOF in their study to isolate and identify THCA and 12 of its metabolites in rat urine using LC-MS, LC-MS/MS, and LC-qTOF MS. The use of qTOF results in increased accuracy of the detected ions, and when analyzing extracts acquired from complex matrices using MS/MS, qTOF allows for increased cannabinoid specificity.[15]
Although MS offers many benefits, the use of qTOF mass spectrometers is ideal when trying to differentiate between two compounds with different compositions but the same nominal mass.[7] qTOF mass spectrometers can provide accurate mass identification with a threshold less than 5 ppm for precursor and product ions; this allows for differentiation between isomers of cannabinoids[15][7] such as Δ8-tetrahydrocannabinol and Δ9-tetrahydrocannabinol, which have the same m/z because these cannot be differentiated by MS.[7] Such isomers may have different therapeutic properties and may need to be separated for manufacture, so it is important to adopt an analytical technique that can differentiate between them.
Matrix-assisted laser desorption/ionization mass spectrometry
Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) is a new method which has been used in some studies for comparison with usual methods such as LC-MS and GC-MS in identification of cannabinoid metabolites.[32] Recently, this method has attracted attention because, when compared to usual mentioned methods, the sample preparation is simpler, a narrower time frame of drug can be detected, and the sample amount is reduced. Beasley et al.[32] have used MALDI-MS to detect the cannabinoids in a single hair sample. In this study, the MALDI instrument consisted of an MDS Sciex hybrid quadrupole time-of-flight mass spectrometer with an orthogonal MALDI ion source and a neodymium-doped yttrium aluminum garnet laser.
LC and GC methods comparison
As stated prior, LC often employs ESI and APCI as ionization sources.[30] These usually only generate a protonated molecule without diagnostic fragmentation; therefore MS/MS is required to obtain diagnostic information when using LC. Additionally, because cannabinoids have phenolic and carboxylic functional groups that are not ionized effectively using ESI or APCI, GC-MS may offer greater sensitivity than LC-MS.[15] GC × GC provides greater separation power and analysis speed compared to coupled-column techniques such as LC-MS.[24]
However, unlike GC, HPLC allows for differentiation between acidic and neutral cannabinoids[21]; it does not require a derivatization step prior to analysis because high temperatures are not involved in the analysis.[5][15] As a result, cannabinoids do not undergo decarboxylation in an HPLC column, so HPLC provides a more comprehensive chemical report of cannabis samples compared to GC. Table 4 shows LOD and LOQ values for some of the most well-known cannabinoids using HPLC and GC-MS.
| ||||||||||||||||||||||||||||||||||||||||
Overall cost is another consideration when comparing these methods. Set-up, maintenance, and running costs are often important factors in selecting analytical techniques, especially in industrial settings. The running cost of LC, GC, and HPLC are negligible, yet equipment-wise, LC is more expensive than GC, and both are much more expensive than HPLC. Coupling of mass spectrometry with LC or GC can further increase the costs.
Additional methods
Thin-layer chromatography
Romano and Hazekamp[21] used the thin-layer chromatography (TLC) method both for polar and non-polar systems. They used reversed-phase silica gel plates and normal-phase silica gel plates for non-polar and polar systems respectively. For more accurate results, they used Fast-Blue B (FBB) salt (4-benzoylamino-2, 5-diethoxy benzene diazonium chloride hemi salt), which is a suitable coloring agent for visualization of cannabinoids on TLC plates. FBB can determine acetylcholinesterase, α- and β-glucosidase activity by changing to different colors, which occurs when FBB reacts with various compounds; however, the colors depend on the concentration of constituents. As a result, they found that TLC is useful in rapid screening of many samples for the existence of cannabinoids. However, its performance is lower compared to other separation methods, and the reproducibility of TLC depends on parameters such as relative humidity.[21]
Fourier-transform infrared spectroscopy
Hazekamp et al.[16] have measured cannabinoids using Fourier-transform infrared spectroscopy (FTIR). They added KBr to the ethanolic solution of cannabinoids, followed by vacuum ethanol evaporation, as KBr does not show any absorption spectrum in the infrared region. Additionally, KBr has a 100% transmission window in the range of wave number with FTIR spectroscopy. The infrared spectra were measured in the range of 500 to 4000 cm−1. Compared to ultraviolet spectra, infrared spectra presented more absorbance peaks.[16] Mutjé et al.[33] offer another example, showing the existence of carbonyl and ester groups by the FTIR peak at 1775 and 1725 cm−1 in composite samples of cannabis extract.
Nuclear magnetic resonance spectrometry
Another alternative to GC and HPLC is nuclear magnetic resonance spectroscopy (NMR).[6][7] NMR is accurate and reproducible, and unlike GS and HPLC, NMR is not sensitive to impurities like chlorophyll or lipids present in the sample.[6] Hazekamp et al.[6] developed a method for cannabinoid quantification using 1H-NMR that does not require chromatographic purification and has a five-minute final analysis time. In that study, they analyzed singlets in the δ 4.0–7.0 range in the 1H-NMR spectrum, and they found that their technique was appropriate for the quantification of CBDA, THCA, CBG, CBGA, and possibly other cannabinoids as well. One of the major advantages of this technique is that reference standards are not required, meaning that this method can quantify cannabinoids that lack pre-existing reference standards and therefore cannot be analyzed by other techniques. Although the results from NMR are promising, one major disadvantage to NMR is that high-resolution instruments are very expensive.[7]
Other parameters to consider when selecting a quantification method
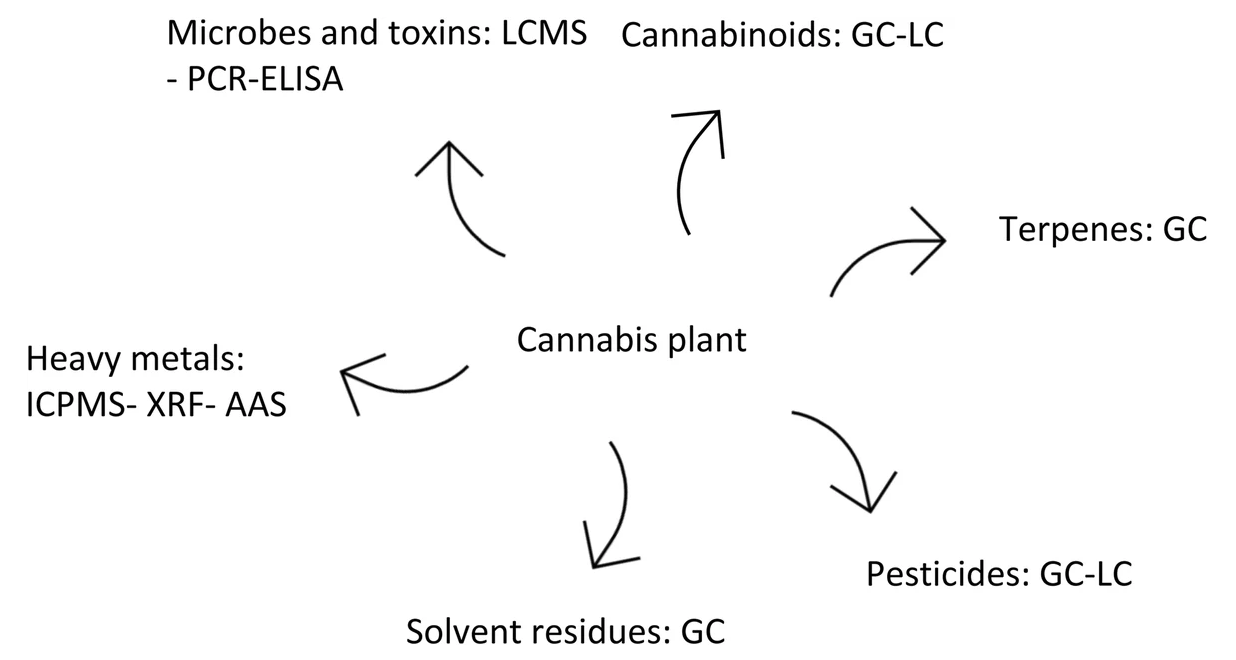
There are other aspects which must be considered when selecting a quantification method, such as method performance for different types of cannabinoids, as well as common interfering substances and impurities. Figure 2 shows suggested methods for cannabis compounds and their impurities. There is no evidence to support which analytical method works best for a specific cannabinoid. However, broadly speaking, LC is the preferred method for cannabinoids and GC the preferred method for terpenes. GC does not have the capability to quantify the acidic form of cannabinoids unless through derivatization, while terpenes cannot be detected by LC because they are volatile compounds. Another important factor to consider is analytical sample preparation, which is time-consuming and the most common cause for generating errors during the analytical process. Sample preparation methods, storage, and handling are some of the procedural concerns which can affect the results. For cannabis, the final sample should represent the original lot. Not only the extraction method, but also the cultivation and processing steps play an important role in the analytical results.
|
Conclusion
Given that cannabis preparations from the same cannabis strain can vary by as much as 25% in cannabinoid composition, there is a clear need to develop an effective and efficient cannabinoid quantification technique so that clinicians can be certain about the chemical properties of the products they are administering.[34] This literature review has explored a variety of cannabinoid quantification techniques.
GC-MS is often employed for cannabinoid quantification.[13][14][25][31][35][36] However, quantification of cannabinoids via GC requires a derivatization step to avoid the decarboxylation of acidic cannabinoids.[1][7][15][30] Performing GC without derivatization requires the calculation of total cannabinoid content from a combination of acidic and neutral cannabinoid content, which can be an uncertain process.[12] HPLC-DAD and HPLC-UV provide alternatives to GC analysis, but these detection techniques lack specificity and sensitivity.[30][37] However, the literature suggests that HPLC-MS/MS using ESI and APCI methods provide enough specificity and sensitivity to quantify cannabinoid content in all cannabis extracts.[5][7][30]
There are multiple benefits to using HPLC-MS/MS over other analytical methods presented in this review. For example, HPLC can differentiate between acidic and neutral cannabinoids, unlike GC.[21] MS offers several benefits over other detection methods as well. For example, MS can differentiate between different cannabinoids based on the m/z value of their molecular ion. It also offers more specificity when compared to ultraviolet detectors and can analyze extracts from complex matrices like cannabis.[7][15]
Abbreviations
Δ8-THC: (−)-Δ8-trans-tetrahydrocannabinol
APCI: Atmospheric-pressure chemical ionization
CBC: Cannabichromene
CBD: Cannabidiol
CBDA: Cannabidiol acid
CBE: Cannabielsoin
CBG: Cannabigerol
CBGA: Cannabigerolic acid
CBL: Cannabicyclol
CBN: Cannabinol
CBND: Cannabinodiol
CBT: Cannabitriol
DAD: Diode array detection
ESI-qTOF: Electrospray ionization-quadrupole-time-of-flight
FBB: Fast-Blue B salt
FID: Flame ionization detection
FTIR: Fourier-transform infrared spectroscopy
GC: Gas chromatography
GC-VUV: Gas chromatography with vacuum ultraviolet spectroscopy
HPLC: High-performance liquid chromatography
LC: Liquid Chromatography
MALDI-MS: Matrix-assisted laser desorption/ionization mass spectrometry
MRM: Multiple reaction monitoring
MS: Mass spectrometry
NMR: Nuclear magnetic resonance spectrometry
THC: Tetrahydrocannabinol
THCA: Tetrahydrocannabinolic acid
THCV: Tetrahydrocannabivarin
TLC: Thin-layer chromatography
UPLC: Ultra-performance liquid chromatography
Acknowledgements
Contributions
Stephanie Torres wrote the skeleton of this paper, and Masoumeh Pourseyed Lazarjani completed it and edited different drafts, which were revised by Owen Young, Ali Seyfoddin, Chris Fowlie, and Thom Hooker. The authors read and approved the final manuscript.
Funding
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 De Backer, B.; Debrus, B.; Lebrun, P. et al. (2009). "Innovative development and validation of an HPLC/DAD method for the qualitative and quantitative determination of major cannabinoids in cannabis plant material". Journal of Chromatography B 877 (32): 4115–24. doi:10.1016/j.jchromb.2009.11.004. PMID 19932642.
- ↑ 2.0 2.1 2.2 2.3 Hillig, K.W.; Mahlberg, P.G. (2004). "A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae)". American Journal of Botany 91 (6): 966-75. doi:10.3732/ajb.91.6.966. PMID 21653452.
- ↑ Knight, G.; Hansen, S.; Connor, M. et al. (2010). "The results of an experimental indoor hydroponic Cannabis growing study, using the 'Screen of Green' (ScrOG) method-Yield, tetrahydrocannabinol (THC) and DNA analysis". Forensic Science International 202 (1–3): 36–44. doi:10.1016/j.forsciint.2010.04.022. PMID 20462712.
- ↑ 4.0 4.1 4.2 4.3 Fischedick, J.T.; Van Der Kooy, F.; Verpoorte, R. (2010). "Cannabinoid receptor 1 binding activity and quantitative analysis of Cannabis sativa L. smoke and vapor". Chemical & Pharmaceutical Bulletin 58 (2): 201–7. doi:10.1248/cpb.58.201. PMID 20118579.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 Aizpurua-Olaizola, O.; Omar, J.; Navarro, P. et al. (2014). "Identification and quantification of cannabinoids in Cannabis sativa L. plants by high performance liquid chromatography-mass spectrometry". Analytical and Bioanalytical Chemistry 406 (29): 7549-60. doi:10.1007/s00216-014-8177-x. PMID 25338935.
- ↑ 6.0 6.1 6.2 6.3 6.4 Hazekamp, A.; Choi Y.H.; Verpoorte, R. (2004). "Quantitative analysis of cannabinoids from Cannabis sativa using 1H-NMR". Chemical and Pharmaceutical Bulletin 52 (6): 718–21. doi:10.1248/cpb.52.718. PMID 15187394.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 7.16 7.17 7.18 7.19 Citti, C.; Braghiroli, D.; Vandelli, M.A. et al. (2018). "Pharmaceutical and biomedical analysis of cannabinoids: A critical review". Journal of Pharmaceutical and Biomedical Analysis 147: 565–79. doi:10.1016/j.jpba.2017.06.003. PMID 28641906.
- ↑ 8.0 8.1 8.2 Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E. et al. (2016). "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products 79 (2): 324-31. doi:10.1021/acs.jnatprod.5b00949. PMID 26836472.
- ↑ Berman, P.; Futoran, K.; Lewitus, G.M. et al. (2018). "A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis". Scientific Reports 8 (1): 14280. doi:10.1038/s41598-018-32651-4. PMC PMC6155167. PMID 30250104. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6155167.
- ↑ Fathordoobady, F.; Singh, A.; Kitts, D.D. et al. (2019). "Hemp (Cannabis sativa L.) Extract: Anti-Microbial Properties, Methods of Extraction, and Potential Oral Delivery". Food Reviews International 35 (7): 664–84. doi:10.1080/87559129.2019.1600539.
- ↑ 11.0 11.1 Fischedick, J.T.; Hazekamp, A.; Erkelens, T. et al. (2010). "Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes". Phytochemistry 71 (17–18): 2058–73. doi:10.1016/j.phytochem.2010.10.001. PMID 21040939.
- ↑ 12.0 12.1 12.2 Dussy, F.E.; Hamberg, C.; Luginbühl, M. et al. (2005). "Isolation of Delta9-THCA-A from hemp and analytical aspects concerning the determination of Delta9-THC in cannabis products". Forensic Science International 149 (1): 3–10. doi:10.1016/j.forsciint.2004.05.015. PMID 15734104.
- ↑ 13.0 13.1 13.2 Omar, J.; Olivares, M.; Amigo, J.M. et al. (2014). "Resolution of co-eluting compounds of Cannabis sativa in comprehensive two-dimensional gas chromatography/mass spectrometry detection with Multivariate Curve Resolution-Alternating Least Squares". Talanta 121: 273–80. doi:10.1016/j.talanta.2013.12.044. PMID 24607138.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 14.7 14.8 14.9 Hazekamp, A.; Simons, R.; Peltenburg-Looman, A. et al. (2004). "Preparative Isolation of Cannabinoids from Cannabis sativa by Centrifugal Partition Chromatography". Journal of Liquid Chromatography & Related Technologies 27 (15): 2421–39. doi:10.1081/JLC-200028170.
- ↑ 15.00 15.01 15.02 15.03 15.04 15.05 15.06 15.07 15.08 15.09 15.10 15.11 15.12 15.13 15.14 Leghissa, A.; Hildenbrand, Z.L.; Schug, K.A. (2018). "A review of methods for the chemical characterization of cannabis natural products". Journal of Separation Science 41 (1): 398-415. doi:10.1002/jssc.201701003. PMID 28986974.
- ↑ 16.0 16.1 16.2 16.3 16.4 Hazekamp, A.; Peltenburg, A.; Verpoorte, R. et al. (2005). "Chromatographic and Spectroscopic Data of Cannabinoids from Cannabis sativa L.". Journal of Liquid Chromatography & Related Technologies 28 (15): 2361–82. doi:10.1080/10826070500187558.
- ↑ 17.0 17.1 Namdar, D.; Mazuz, M; Ion, A. et al. (2018). "Variation in the compositions of cannabinoid and terpenoids in Cannabis sativa derived from inflorescence position along the stem and extraction methods". Industrial Crops and Products 113: 376–82. doi:10.1016/j.indcrop.2018.01.060.
- ↑ 18.0 18.1 Casiraghi, A.; Roda, G.; Casagni, E. et al. (2018). "Extraction Method and Analysis of Cannabinoids in Cannabis Olive Oil Preparations". Planta Medica 84 (4): 242-249. doi:10.1055/s-0043-123074. PMID 29202510.
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 Pellati, F.; Brighenti, V.; Sperlea, J. et al. (2018). "New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (hemp)". Molecules 23 (10): 2639. doi:10.3390/molecules23102639. PMC PMC6222702. PMID 30322208. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6222702.
- ↑ 20.0 20.1 Namdar, D.; Charuvi, D.; Ajjampura, V. et al. (2019). "LED lighting affects the composition and biological activity of Cannabis sativa secondary metabolites". Industrial Crops and Products 132: 177–85. doi:10.1016/j.indcrop.2019.02.016.
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 Romano, L.L.; Hazekamp, A. (2013). "Cannabis oil: Chemical evaluation of an upcoming cannabis-based medicine". Cannabinoids 1 (1): 1–11. https://www.cannabis-med.org/index.php?tpl=cannabinoids&id=276&lng=en&red=cannabinoidslist.
- ↑ Leghissa, A.; Hildenbrand, Z.L.; Foss, F.W. et al. (2018). "Determination of cannabinoids from a surrogate hops matrix using multiple reaction monitoring gas chromatography with triple quadrupole mass spectrometry". Journal of Separation Science 41 (2): 459–68. doi:10.1002/jssc.201700946. PMID 29094798.
- ↑ Leghissa, A.; Smuts, J.; Qiu, C. et al. (2018). "Detection of cannabinoids and cannabinoid metabolites using gas chromatography with vacuum ultraviolet spectroscopy". Separation Science Plus 1 (1): 37–42. doi:10.1002/sscp.201700005.
- ↑ 24.0 24.1 24.2 Dallüge, J.; Beens, J.; Th Brinkman, U.A. (2003). "Comprehensive two-dimensional gas chromatography: a powerful and versatile analytical tool". Journal of Chromatography A 1000 (1–2): 69–108. doi:10.1016/s0021-9673(03)00242-5.
- ↑ 25.0 25.1 Gröger, T.; Schäffer, M.; Pütz, M. et al. (2008). "Application of two-dimensional gas chromatography combined with pixel-based chemometric processing for the chemical profiling of illicit drug samples". Journal of Chromatography A 1200 (1): 8–16. doi:10.1016/j.chroma.2008.05.028. PMID 18539290.
- ↑ 26.0 26.1 26.2 26.3 26.4 26.5 Citti, C.; Ciccarella, G.; Braghiroli, D. et al. (2016). "Medicinal cannabis: Principal cannabinoids concentration and their stability evaluated by a high performance liquid chromatography coupled to diode array and quadrupole time of flight mass spectrometry method". Journal of Pharmaceutical and Biomedical Analysis 128: 201–09. doi:10.1016/j.jpba.2016.05.033. PMID 27268223.
- ↑ 27.0 27.1 Peschel, W.; Politi, M. (2015). "¹H NMR and HPLC/DAD for Cannabis sativa L. chemotype distinction, extract profiling and specification". Talanta 140: 150–65. doi:10.1016/j.talanta.2015.02.040. PMID 26048837.
- ↑ 28.0 28.1 28.2 28.3 Brighenti, V.; Pellati, F.; Steinbach, M. et al. (2017). "Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp)". Journal of Pharmaceutical and Biomedical Analysis 143: 228–36. doi:10.1016/j.jpba.2017.05.049. PMID 28609672.
- ↑ Oomah, B.D.; Busson, M.; Godfrey, D.V. et al. (2002). "Characteristics of hemp (Cannabis sativa L.) seed oil". Fod Chemistry 76 (1): 33–43. doi:10.1016/S0308-8146(01)00245-X.
- ↑ 30.0 30.1 30.2 30.3 30.4 30.5 Grauwiler, S.B.; Scholer, A.; Drewe, J. (2007). "Development of a LC/MS/MS method for the analysis of cannabinoids in human EDTA-plasma and urine after small doses of Cannabis sativa extracts". Journal of Chromatogrophy B 850 (1–2): 515–22. doi:10.1016/j.jchromb.2006.12.045. PMID 17236827.
- ↑ 31.0 31.1 Jung, J.; Meyer, M.R.; Maurer, H.H. et al. (2009). "Studies on the metabolism of the Delta9-tetrahydrocannabinol precursor Delta9-tetrahydrocannabinolic acid A (Delta9-THCA-A) in rat using LC-MS/MS, LC-QTOF MS and GC-MS techniques". Journal of Mass Spectrometry 44 (10): 1423–33. doi:10.1002/jms.1624. PMID 19728318.
- ↑ 32.0 32.1 Beasley, E.; Francese, S.; Bassindale, T. (2016). "Detection and Mapping of Cannabinoids in Single Hair Samples through Rapid Derivatization and Matrix-Assisted Laser Desorption Ionization Mass Spectrometry". Analytical Chemistry 88 (20): 10328-10334. doi:10.1021/acs.analchem.6b03551. PMID 27648476.
- ↑ Mutjé, P.; Lòpez, A.; Vallejos, M.E. et al. (2007). "Full exploitation of Cannabis sativa as reinforcement/filler of thermoplastic composite materials". Composites Part A 38 (2): 369–77. doi:10.1016/j.compositesa.2006.03.009.
- ↑ Hazekamp, A.; Fischedick, J.T. (2012). "Cannabis - From cultivar to chemovar". Drug testing and analysis 4 (7–8): 660–7. doi:10.1002/dta.407. PMID 22362625.
- ↑ Omar, J.; Olivares, M.; Alzaga, M. et al. (2013). "Optimisation and characterisation of marihuana extracts obtained by supercritical fluid extraction and focused ultrasound extraction and retention time locking GC-MS". Journal of Separation Science 36 (8): 1397–404. doi:10.1002/jssc.201201103. PMID 23505258.
- ↑ Ciolino, L.A.; Ranieri, T.L.; Taylor, A.M. (2018). "Commercial cannabis consumer products part 1: GC-MS qualitative analysis of cannabis cannabinoids". Forensic Science International 289: 429–37. doi:10.1016/j.forsciint.2018.05.032. PMID 29954632.
- ↑ Galal, A.M.; Slade, D.; Gul, W. et al. (2009). "Naturally occurring and related synthetic cannabinoids and their potential therapeutic applications". Recent Patents on CNS Drug Discovery 4 (2): 112–36. doi:10.2174/157488909788453031. PMID 19519560.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. The original article lists references in alphabetical order; this wiki organizes them by order of appearance, by design. Several of the original citations had incorrect publication years; they were corrected for this version. One ref (Aminah Jatoi et al. 2002) didn't fit into the context of the article and appeared to be added in error; the presumably correct citation for Citti 2016 was used in its place for this version. A similar error was found for a Peschel and Politi citation (using Andreae et al. 2015), a Citti et al. 2018 citation (using Beal et al. 1995), a Aizpurua-Olaizola et al. citation (using Borgelt et al. 2013), and a Jung et al. citation (using Brighenti et al. 2017). In all these cases, for this version the proper citation was used, and the irrelevant citations (in parentheses) were not loaded or used.