Journal:The effect of a test ordering software intervention on the prescription of unnecessary laboratory tests - A randomized controlled trial
| Full article title | The effect of a test ordering software intervention on the prescription of unnecessary laboratory tests - A randomized controlled trial |
|---|---|
| Journal | BMC Medical Informatics and Decision Making |
| Author(s) |
Martins, C.M.; da Costa Teixeira, A.S.; de Azevedo. L.F.; Sá, L.M.; Santos, P.A.; do Couto, M.L.; da Costa Pereira, A.M.; Hespanhol, A.A.; da Costa Santos, C.M. |
| Author affiliation(s) | University of Porto |
| Primary contact | E-mail: carlosmartins20 at gmail dot com |
| Year published | 2017 |
| Volume and issue | 17 (1) |
| Page(s) | 20 |
| DOI | 10.1186/s12911-017-0416-6 |
| ISSN | 1472-6947 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-017-0416-6 |
| Download | https://bmcmedinformdecismak.biomedcentral.com/track/pdf/10.1186/s12911-017-0416-6 (PDF) |
|
|
This article should not be considered complete until this message box has been removed. This is a work in progress. |
Abstract
Background: The way electronic health record and laboratory test ordering system software is designed may influence physicians’ prescription. A randomized controlled trial was performed to measure the impact of a diagnostic and laboratory tests ordering system software modification.
Methods: Participants were family physicians working and prescribing diagnostic and laboratory tests.
The intervention group had modified software with basic shortcut menu changes, where some tests were withdrawn or added, and with the implementation of an evidence-based clinical decision support system based on United States Preventive Services Task Force (USPSTF) recommendations. This intervention group was compared with typically used software (control group).
The outcomes were the number of tests prescribed from those: withdrawn from the basic menu; added to the basic menu; marked with green dots (USPSTF’s grade A and B); and marked with red dots (USPSTF’s grade D).
Results: Comparing the monthly average number of tests prescribed before and after the software modification, from those tests that were withdrawn from the basic menu, the control group prescribed 33.8 tests per 100 consultations before and 30.8 after (p = 0075); the intervention group prescribed 31.3 before and 13.9 after (p < 0001). Comparing the tests prescribed between both groups during the intervention, from those tests that were withdrawn from the basic menu, the intervention group prescribed a monthly average of 14.0 vs. 29.3 tests per 100 consultations in the control group (p < 0.001). From those tests that are USPSTF’s grade A and B, the intervention group prescribed 66.8 vs. 74.1 tests per 100 consultations in the control group (p = 0.070). From those tests categorized as USPSTF grade D, the intervention group prescribed an average of 9.8 vs. 11.8 tests per 100 consultations in the control group (p = 0.003).
Conclusions: Removing unnecessary tests from a quick shortcut menu of the diagnosis and laboratory tests ordering system had a significant impact and reduced unnecessary prescription of tests.
The fact that it was not possible to perform the randomization at the family physicians’ level, but only on the computer servers is a limitation of our study. Future research should assess the impact of different test ordering systems during longer periods.
Trial registration: ISRCTN45427977, May 1st 2014 (retrospectively registered).
Keywords: Preventive health services, primary health care, evidence-based practice, decision support systems, clinical decision making, computer-assisted
Background
Informatics has undoubtedly changed the way societies live, socialize, learn, work, and deal with healthcare. We now live in a period of increasing concern about the excessive presence of medicine in our lives.[1][2][3] When inefficient software is combined with a non-evidence-based medical practice, there is the risk of patient harm, significant impact to quality of life, and damage to the healthcare system due to unnecessary costs.
The implementation of electronic health records (EHRs) has both potential benefits and drawbacks.[4] Among the benefits, the prevention of medical errors and the promotion of patient safety has often been mentioned and confirmed in clinical practice.[4][5][6] Despite the positive effects of EHR implementation in clinical practice, a range of barriers faced by physicians has been identified. These barriers may include technical and financial aspects, time, psychological, social, legal, and organizational changes to the process.[7] After having removed the first barriers to EHR implementation, it is now time to implement continuing improvement and development of the available tools and to incorporate the scientific evidence obtained to this point.[4][8][9][10]
To achieve better patient safety standards and improve healthcare system cost-effectiveness, there has been a worldwide effort to implement an integrated EHR system with diagnostic and laboratory test ordering communication systems.[11][12][13] There have also been attempts to incorporate clinical decision support systems to further improve the quality of medicine. Prescribing diagnostic and laboratory tests is a key component of medical consultation. In the primary health care setting, tests are often ordered with preventive intentions and fulfillment of patient expectations.[14][15] There is also great uncertainty and variability among family physicians’ ordering routines.[16][17][18] The effects of test ordering communication systems integrated with clinical decision support systems have been reported in various clinical practice settings. Main et al. have performed a systematic review of this topic and reported that integration of clinical decision support systems resulted in significant benefits to the prescribing process and practitioner performance outcomes in nearly two-thirds of the 24 studies that met the inclusion criteria.[19]
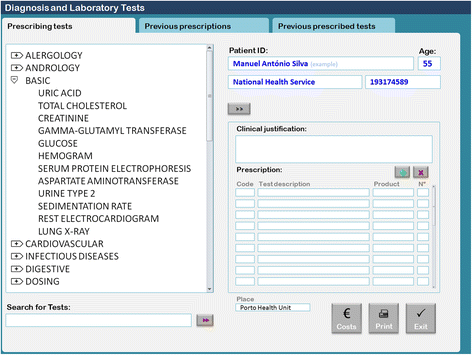
In Portugal, the use of EHR software with a diagnostic and laboratory test order communication system has been mandatory since September 2011. Most of the primary healthcare centers use software called Sistema de Apoio ao Médico (Physician’s Support System [SAM]). In the module used to order diagnostic and laboratory tests, physicians access a searchable test menu by two possible strategies: 1) typing the test name in a search box or 2) browsing by a shortcut menu structure (Fig. 1). Different menus are available for most areas of medicine, including basic, allergology, andrology, cardiovascular, central nervous system, digestive, dosing, endocrinology, gynecology, hematology, infectious diseases, nephrology, obstetrics, oncology, otorhinolaryngology, osteoarticular, preoperative, respiratory, rheumatology, and urology. Under each menu there is a set of specific lab tests. Physicians can choose one or more tests by double-clicking each test or can choose the entire set by double-clicking on the shortcut menu’s title. For example, the basic menu is composed of uric acid, total cholesterol, creatinine, gamma-glutamyl transferase, glucose, hemogram, serum protein electrophoresis, aspartate aminotransferase, urine type 2, sedimentation rate, electrocardiogram, and lung x-ray tests.
|
If a physician would like to choose only hemogram and glucose, they must double-click on each test. However, if they would like the entire basic set of tests, they must double-click on the basic menu title. Our research team suspected that this basic shortcut menu is often selected during routine consultations in which patients ask for routine check-ups without specific reason. As we have shown in a previous study, there is a high prevalence of Portuguese adults (99.2%) that believe they should have routine blood and urine tests annually.[14] This statistic demonstrates the importance of examining the effectiveness and efficiency of this basic sub-menu.
Through a randomized controlled trial, the primary aim of the present study was to compare the effects of modifying the EHR ordering communication system (modified SAM) by changing the basic shortcut menu and adding a clinical decision support system based on the integration of the United States Preventive Services Task Force (USPSTF) recommendations. After the last primary healthcare reform in Portugal, primary healthcare centers have been divided into healthcare center groups.[20] In a healthcare center group, the informatics network is linked through servers that may serve more than one healthcare center. Creating a modified version of the SAM software requires it to be installed at a server level, which determines that all physicians at all healthcare centers served by that server will receive the same version of the software. For this reason, it was not possible to randomize the study at the physician level. Rather, we had to randomize the servers at the healthcare center group level.
Methods
Trial design
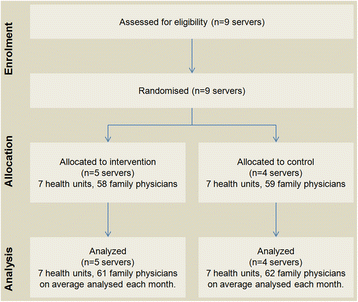
All servers of the Western Oporto grouping of health centers counted for randomization, except that serving the center where study authors worked (to avoid possible bias). The remaining nine servers were randomized into two groups: 1) five servers were randomly allocated to the intervention group and 2) four servers to the control group.
Participants
All family physicians working and prescribing diagnostic and laboratory tests in the Western Oporto group of health centers (except in those where the authors worked) participated in this study.
Data of the diagnostic and laboratory tests prescriptions were centrally collected by informatics staff belonging to the Ministry of Health and sent to the research team without patients’ or physicians’ identifications.
Interventions
The control group continued to use the standard version of the EHR software (SAM) as presented in Fig. 1. The intervention group used a modified version of the software (SAM modified) installed in each server (Fig. 2). Software modifications consisted of two principal changes:
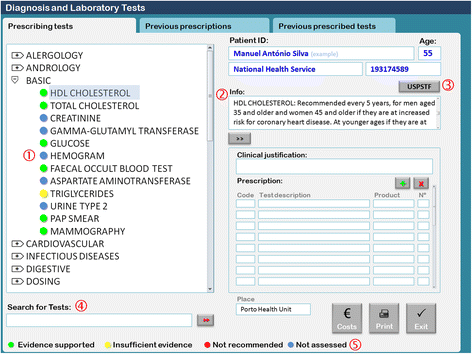
1. Basic shortcut menu changes: The composition of the basic menu set of diagnostic laboratory tests was changed. Some tests were removed, including uric acid, serum protein electrophoresis, sedimentation rate, electrocardiogram, and lung x-ray. Other tests were added, including HDL cholesterol, fecal occult blood test, triglycerides, Pap smear, and mammography. Although some tests were removed from the basic menu, physicians were still able to request them by typing their names in the search for tests box.
2. Addition of an evidence-based decision support: For the tests listed in Table 1, we added traffic light-based colored dots according to the USPSTF recommendations and an additional information box containing the summary of the USPSTF recommendation and a link to the integral recommendation at the USPSTF website (Fig. 2).[21]
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The selection of the specific tests to be removed from or added to the basic shortcut menu was made after discussion and debate with eight family physicians selected by proximity and convenience. The main rational was to remove tests that are not recommended to be used as routine screening tools and to substitute them with others that may be recommended for certain risk groups. However, creating a change that was not too disruptive to the current family physicians’ practices was a concern, and that is why tests such as creatinine, gamma-glutamyl transferase, hemogram, aspartate aminotransferase, and urine type 2 were left in the basic shortcut menu.
EHR software modifications were implemented on May 30–31, 2012 in all of the intervention groups’ servers. Prospective monthly monitoring and data collection occurred until January 31, 2013. To allow a pre-post analysis in both groups, a retrospective monthly data collection of both control and intervention groups was also performed between December 1, 2011 and May 31, 2012.
The collected data included monthly parameters, including numbers of prescribing family physicians, face-to-face consultations made, and number of each prescribed diagnostic and laboratory test.
Independent variables
Group (control and intervention) and time (before and after the modifications) were the independent variables.
Dependent variables
Primary outcomes were chosen to assess the impact of our intervention on the number of diagnostic and laboratory tests prescribed by physicians using four different perspectives: 1) impact on the number of the prescriptions of diagnostic and laboratory tests that were withdrawn from the basic menu; 2) impact on the number of the prescriptions of diagnostic and laboratory tests that were added to the basic menu; 3) impact on the number of the prescriptions of diagnostic and laboratory tests that were marked with green dots (USPSTF recommendation grades A and B); and 4) impact on the number of prescriptions of diagnostic and laboratory tests that were marked with red dots (USPSTF recommendation grade D). Diagnostic and laboratory tests were the dependent variables.
Sample size
Given administrative and technical barriers related to the permission process for the introduction of EHR software modifications, our sample size was obtained by convenience. We decided to run this trial at the Western Oporto grouping of healthcare centers, which includes a total of 15 primary care healthcare centers and an informatics network of 10 servers. One of the servers, which served the healthcare center in which some of the study authors worked, was excluded from the study.
The power calculation was done for the mean number of monthly prescribed tests per 100 consultations comparison between control and intervention groups. A significance level of 0.05, a power of 0.8, and a standard deviation of two tests prescribed per 100 consultations per month was considered. A mean difference of 3.3 tests prescribed per 100 consultations per month between control and intervention groups was used.
Randomization
The remaining nine servers were sequentially numerated and randomly assigned to the intervention and control group by an investigator blinded to the server identification. The allocation sequence was computer generated resulting in five servers (seven healthcare centers, 58 family physicians) allocated to the intervention group, and four servers (seven healthcare centers, 59 family physicians) allocated to the control group.
To guarantee allocation anonymity, family physicians at each healthcare center only received information about this trial implementation after randomization has been performed. Given the nature of this trial, no consent at physician level was obtained. Consent was obtained from the Northern Regional Health Administration and the Executive Council of the Western Oporto Group of Health Centers.
Statistical methods
To examine whether the software modification changed test prescription trends we performed an interrupted time series analysis with an autoregressive integrated moving average (ARIMA) model, using the monthly number of tests prescribed per 100 consultations and the intervention (the software modification) as a dichotomous variable (before and after intervention). In the control group, we also performed an ARIMA model analysis using the monthly number of tests prescribed per 100 consultations and the same dichotomous variable (before and after intervention), although there was no intervention in this group. The before and after intervention comparison of the monthly average number of diagnostic and lab tests prescribed per 100 consultations between control and intervention groups was made using an independent sample t test. The Bonferroni correction was used for adjusting for multiple testing (several diagnostic and laboratory tests).[22] A significance level of 0.05 was used. As size effect measure, Cohen’s d was calculated and a d of ≥0.8 was considered a large effect.
Ethical considerations
This study was approved by the Northern Regional Health Administration Medical Ethics Committee with several considerations: “1) Given that the study will collect only anonymous data there is no need for the obtainment of informed consent by physicians; 2) The study is of great relevance and with an expected practical interest of the results; and 3) The methodology used safeguards the rights of participants.”
In accordance with the Medical Ethics Committee, before the implementation of the study (but after the randomized allocation), a letter was sent to all Western Oporto Group of healthcare centers informing them of and explaining the study aims and methodology.
Results
The number of servers enrolled was constant without loss (Fig. 3). The number of family physicians after the intervention ranged from 58 to 64 in the intervention group and from 55 to 70 in the control group. Recruitment was performed in April 2012, and the trial began on June 1, 2012, with a follow-up period until January 31, 2013 (eight month follow-up). Baseline data are presented in Table 2.
|
| ||||||||||||||||||||
For both groups, Table 3 compares the monthly average number of diagnostic and laboratory tests prescribed for each 100 consultations before and after EHR software modification, under a time series analysis perspective (ARIMA model). Within the five tests that were withdrawn from the basic menu, we observe a statistically significant reduction in the prescription of four tests (uric acid, serum protein electrophoresis, sedimentation rate, and lung x-ray tests) in the intervention group after software modification. Within the five tests that were added to the basic menu, we observed a significant increase in prescription of the fecal occult blood test in the intervention group after software modification. Within the tests that were marked with green dots (USPSTF recommendation grades A and B) there were no significant increases in either the control or intervention groups. Within the tests that were marked with red dots (USPSTF recommendation grade D), there was a significant reduction only in the prescription of cancer antigen 19-9, but the amount of this test that was prescribed was so low that this variation may be justified by other factors and not a result of software modification.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- ↑ Glasziou, P.; Moynihan, R.; Richards, T. et al. (2013). "Too much medicine; too little care". BMJ 347: f4247. doi:10.1136/bmj.f4247. PMID 23820022.
- ↑ Moynihan, R.; Doust, J.; Henry, D. (2012). "Preventing overdiagnosis: How to stop harming the healthy". BMJ 344: e3502. doi:10.1136/bmj.e3502. PMID 22645185.
- ↑ Getz, L.; Sigurdsson, J.A.; Hetlevik, I. (2003). "Is opportunistic disease prevention in the consultation ethically justifiable?". BMJ 327 (7413): 498–500. doi:10.1136/bmj.327.7413.498. PMC PMC188390. PMID 12946974. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC188390.
- ↑ 4.0 4.1 4.2 Raposo, V.L. (2015). "Electronic health records: Is it a risk worth taking in healthcare delivery?". GMS Health Technology Assessment 11: Doc02. doi:10.3205/hta000123. PMC PMC4677576. PMID 26693253. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4677576.
- ↑ Ben-Assuli, O. (2015). "Electronic health records, adoption, quality of care, legal and privacy issues and their implementation in emergency departments". Health Policy 119 (3): 287–97. doi:10.1016/j.healthpol.2014.11.014. PMID 25483873.
- ↑ Ben-Assuli, O.; Leshno, M. (2016). "Assessing electronic health record systems in emergency departments: Using a decision analytic Bayesian model". Health Informatics Journal 22 (3): 712–29. doi:10.1177/1460458215584203. PMID 26033468.
- ↑ Boonstra, A.; Broekhuis, M. (2010). "Barriers to the acceptance of electronic medical records by physicians from systematic review to taxonomy and interventions". BMC Health Services Research 10: 231. doi:10.1186/1472-6963-10-231. PMC PMC2924334. PMID 20691097. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2924334.
- ↑ Roukema, J.; Los, R.K.; Bleeker, S.E. (2006). "Paper versus computer: Feasibility of an electronic medical record in general pediatrics". Pediatrics 117 (1): 15–21. doi:10.1542/peds.2004-2741. PMID 16396855.
- ↑ Bowman, S. (2013). "Impact of electronic health record systems on information integrity: Quality and safety implications". Perspectives in Health Information Management 10: 1c. PMC PMC3797550. PMID 24159271. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3797550.
- ↑ Nguyen, L.; Bellucci, E.; Nguyen, L.T. (2014). "Electronic health records implementation: An evaluation of information system impact and contingency factors". International Journal of Medical Informatics 83 (11): 779–96. doi:10.1016/j.ijmedinf.2014.06.011. PMID 25085286.
- ↑ Moen, A.; Hackl, W.O.; Hofdijk, J. et al. (2013). "eHealth in Europe - Status and challenges". Yearbook of Medical Informatics 8: 59–63. PMID 23974549.
- ↑ Olsson, S.; Lymberis, A.; Whitehouse, D. (2004). "European Commission activities in eHealth". International Journal of Circumpolar Health 63 (4): 310-6. PMID 15709306.
- ↑ Iakovidis, I.; Purcarea, O. (2008). "eHealth in Europe: From vision to reality". Studies in Health Technology and Informatics 134: 163–8. PMID 18376043.
- ↑ 14.0 14.1 Martins, C.; Azevedo, L.F.; Ribeiro, O. (2013). "A population-based nationwide cross-sectional study on preventive health services utilization in Portugal - What services (and frequencies) are deemed necessary by patients?". PLOS One 8 (11): e81256. doi:10.1371/journal.pone.0081256. PMC PMC3836775. PMID 24278405. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3836775.
- ↑ van Bokhoven, M.A.; Pleunis-van Empel, M.C.; Koch, H. et al. (2006). "Why do patients want to have their blood tested? A qualitative study of patient expectations in general practice". BMC Family Practice 7: 75. doi:10.1186/1471-2296-7-75. PMC PMC1769380. PMID 17166263. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1769380.
- ↑ van der Weijden, T.; van Bokhoven, M.A.; Dinant, G.J. et al. (2002). "Understanding laboratory testing in diagnostic uncertainty: A qualitative study in general practice". British Journal of General Practice 52 (485): 974–80. PMC PMC1314466. PMID 12528582. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1314466.
- ↑ van Bokhoven, M.A.; Koch, H.; Dinant, G.J. et al. (2008). "Exploring the black box of change in improving test-ordering routines". Family Practice 25 (3): 139-45. doi:10.1093/fampra/cmn022. PMID 18535302.
- ↑ Verstappen, W.H.; ter Riet, G.; Dubois, W.I. et al. (2004). "Variation in test ordering behaviour of GPs: Professional or context-related factors?". Family Practice 21 (4): 387-95. doi:10.1093/fampra/cmh408. PMID 15249527.
- ↑ Main, C.; Moxham, T.; Wyatt, J.C. et al. (2010). "Computerised decision support systems in order communication for diagnostic, screening or monitoring test ordering: Systematic reviews of the effects and cost-effectiveness of systems". Health Technology Assessment 14 (48): 1–227. doi:10.3310/hta14480. PMID 21034668.
- ↑ da Costa Pereira, A.; Giest, S.; Dumortier, J.; Artmann, J. (October 2010). "eHealth Strategies - Country Brief: Portugal" (PDF). European Commission. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.476.4177&rep=rep1&type=pdf.
- ↑ U.S. Preventive Services Task Force (2014). "The Guide to Clinical Preventive Services 2014". https://www.overdrive.com/media/2062549/clinical-preventive-services-2014. Retrieved 08 October 2016.
- ↑ Martins, C.; Azevedo, L.F.; Santos, C. (2014). "Preventive health services implemented by family physicians in Portugal - A cross-sectional study based on two clinical scenarios". BMJ Open 4 (5): e005162. doi:110.1136/bmjopen-2014-005162. PMC PMC4039810. PMID 24861550. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4039810.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. In some cases important information was missing from the references, and that information was added. A significant amount of grammar edits were made to make the document more readable.