LII:The Application of Informatics to Scientific Work: Laboratory Informatics for Newbies
Title: The Application of Informatics to Scientific Work: Laboratory Informatics for Newbies
Author for citation: Joe Liscouski, with editorial modifications by Shawn Douglas
License for content: Creative Commons Attribution-ShareAlike 4.0 International
Publication date: April 2021
Introduction
The purpose of this piece is to introduce people who are not intimately familiar with laboratory work to the basics of laboratory operations and the role that informatics can play in assisting scientists, engineers, and technicians in their efforts. The concepts are important because they provide a functional foundation for understanding lab work and how that work is done in the early part of the twenty-first century (things will change, just wait for it).
Intended audience
This material is intended for anyone who is interested in seeing how modern informatics tools can help those doing scientific work. It will provide an orientation to scientific and laboratory work, as well as the systems that have been developed to make that work more productive. It’s for people coming out of school who have carried out lab experiments but not corporate research projects, for those who need to understand how testing labs work, and for IT professionals who may be faced with supporting computing systems in lab environments. It’s also for those who may be tasked with managing projects to choose, install, and make informatics tools useful.
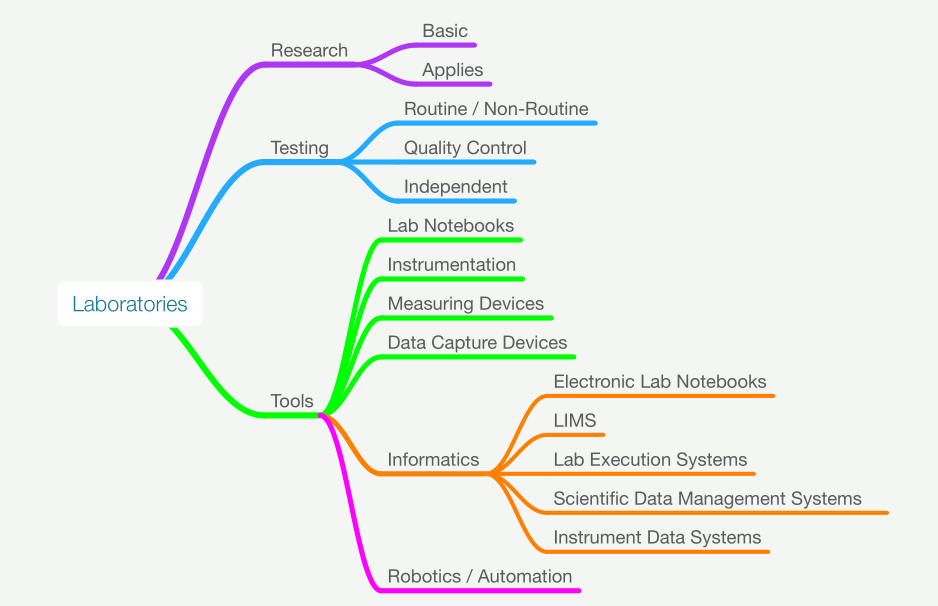
Figure 1 shows the elements we’ll be discussing in this piece. The treatment of the technical material will be on the lighter side, leaving in-depth subject matter to other works. Instrument data systems will be covered lightly, as any serious discussion becomes lengthy and discipline-specific very quickly; additionally, that material has been covered in other works.
|
Types of scientific and laboratory work
Science is about seeking truthful answers to questions. Sometimes those questions are open-ended without any idea where they will lead you in answering them (e.g. “Why does water ice float?”). Others are very specific, concerning material composition or properties (e.g., “How much lead is in this drinking water?”, “How much does a butterfly weigh?”). Still others may take some effort before you determine the best approach to working on them. The approach someone uses to address these questions depends on the nature of the question; some are destined for research, while others are addressed using specific test methods.
There are two types of research: basic and applied. Both can include field work, observations, experiments, models (mathematical, computer, and simulation), etc. Applied research is also done in testing or service laboratories, as with, for example, the development of new methods of analysis.
Basic and applied research
Basic research is open-ended, as you are looking into something without any idea of where the work will lead. It is often funded by grants through universities or government institutions; continued support depends on the perceived value of the research. Projects can range in size from the work of a single individual to a small team to large-scale groups studying astronomy, high-energy physics, engineering, the life sciences, or a number of fields.
Applied research, on the other hand, is directed toward a goal. That goal could be a cure for a disease, the development of a COVID-19 vaccine, or work towards artificial intelligence (AI). As with basic research, until some early goals have been reached the work may begin with a single individual or a small team, and then the project scales up. The effort may be broken down into a set of more narrowly focused efforts, whose results will be combined as the development proceeds. Since applied research is goal-directed, funding will depend upon who benefits from those goals being met. Projects of national interest, including security, may be wholly or partially funded by the government. Projects with a commercial interest tend to be funded by corporate interests, including individual companies in their own laboratories or through contract research organizations with expertise useful to the program. Where there is interest from a number of corporate and/or government groups, consortiums may form to distribute the cost and share in the results.
Both basic and applied research can be found in government institutions (including military groups, research and development agencies like the Defense Advanced Research Project Agency [DARPA], and task-specific institutions such as the National Institutes of Health [NIH]), public and private non-profit groups, corporations, consortia, and contract research organizations.
The research process
The research process begins with a question. Any question will do, including “why is the sky blue?” We’ll bypass Greek mythology[a] by asking more questions and planning how to proceed to answer them. For example, “Is the sky always blue?”, “When is/isn’t it?”, and “What other colors can it be?” Once the process begins, it can include a number of steps, the choice and direction depending upon the nature of the research and the mindset of the researcher:
- Observations: This includes basic note-taking with support material (text, photos, drawings, charts, and scanned material). Research (e.g., as with basic astronomy, field biology) can be as simple as looking something up on Google or as complex as understanding how a virus works. Research is about asking questions and looking for answers, which often leads to more questions. It’s a little like my granddaughter who always asks “why?” no matter how well I answer the previous question (or at least how well I think I did).
- Enhanced observations: This includes interacting with items under observation, as well as non-directed interactions, preliminary data gathering, and behavioral analysis.
- Experiments and information gathering: This includes organized experiments that are planned, directed, and purpose-driven, as well as data and information gathering.
- Team building: This includes the creation of teams or networks of people working on the same or similar projects.
- Analytics and reporting: This includes data and information analysis, data modeling (e.g., mathematical, computer algorithm, and simulation), information synthesis, and knowledge creation.
- Technology acquisition: This includes gaining access to public, commercial, remote, and other types of databases to assist the research.
Pinning down a “typical” approach to research isn’t possible because the routes people follow are as individual as the researchers and their area of work are. However, this is generally not the case with testing labs.
Testing laboratories
In addition to research labs, there are also testing or "service" laboratories. Service labs carry out specific test routines on samples and specimens; you may be familiar with them as quality control labs, clinical labs, toxicology labs, forensic science labs, etc. They are called service labs because they support other organizations, including research organizations, and they have similar modes of operation and work organization, running different tests depending on their area of specialization.
Contract testing labs are another flavor of service laboratories, acting as independent labs that do testing for a fee. These labs can offer capabilities and expertise that their customer doesn’t have, either because the equipment is specialized and not frequently needed or because the customer is looking for a second opinion on an analysis.
Regardless of the type of service lab, they all have one thing in common: the way they function. For a moment let’s forget about science and think about something else. Take for example a company that does graphics printing as a service to graphic designers and marketing groups. The company could offer a variety of printing services:
- business cards
- stationery (e.g., envelopes, letterhead, etc.)
- postcards
- brochures
- signs
- graphics for trade shows (including mounting of an image on backing, lightboxes, etc.)
- postal marketing services
In this scenario, customers can come into the shop and drop off work to be done or place orders online (the company website provides a good description of their services and products). One of their biggest concerns is workflow management: what work is coming in, what is in progress, what is in quality control, and what is ready for delivery. Many activities may be associated with this workflow.
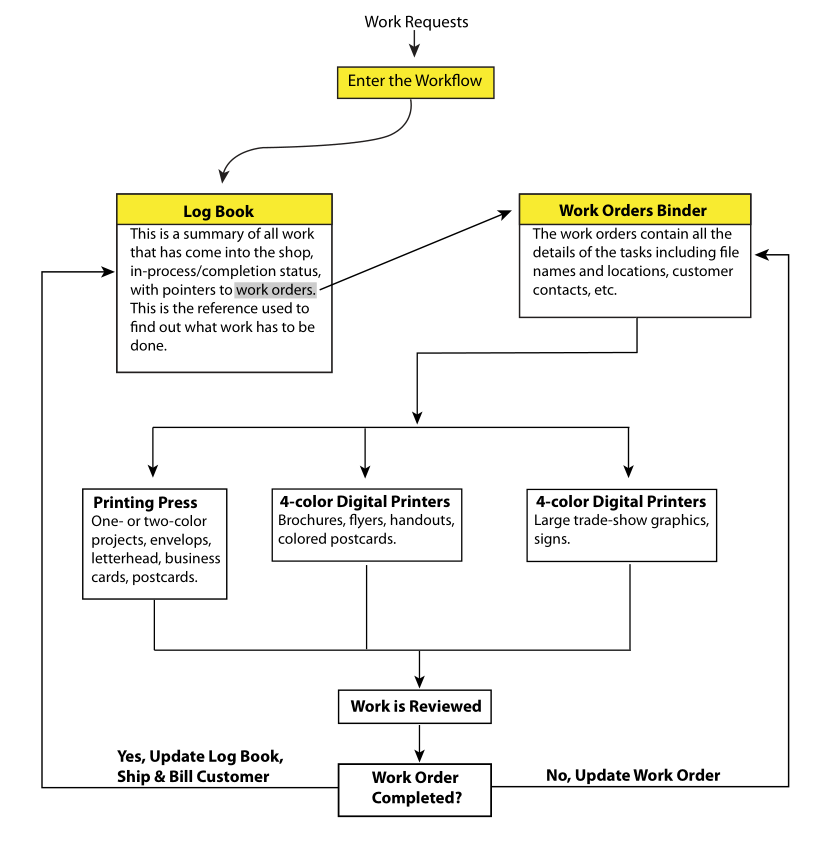
- Order acceptance: Log the job into a log book, the go-to reference for work orders. Add the corresponding work order to a binder of work orders; work orders can be removed from the binders as needed (for example, when people are working on the job) and returned when completed. Comments are made on the work order and referenced to an individual’s notebook for details. Work orders shouldn’t be duplicated since people may not be aware of the duplicates and information may be lost. This does add some inefficiency to the process if a work order contains multiple components (e.g., brochures and trade show graphics); if someone needs to work on a task and someone else has the work order, they have to find it. Work orders contain the names of graphics files and their location. Then a check is made to ensure all the needed information is there, notifying people if something is missing. This includes checking to see if the graphics files are available, in the correct format, etc. The priority of the work is determined with respect to other work. Then the customer is notified of the work order status and the expected completion date.
- Scheduling: The work order is assigned to one or more individuals for completion.
- Performing the work: This is where the actual work is performed, including task coordination if an order has multiple components.
- Customer service: This includes answering customer questions about the work order and addressing inquiries on completion date.
- Draft review: This involves obtaining customer sign-off on a prototype stage if required, making adjustments if needed, and then proceeding to completion.
- Quality control: This is where projects are reviewed and approved for completion.
- Delivery: This involves shipping the material back to the customer or notifying the customer the order is ready for pick-up.
- Billing: After satisfaction with the completed work is acknowledged, the work order is billed to the customer.
When the shop has a large number of projects going on, such a manual, paper-based workflow is difficult and time-consuming to manage. Projects have to be scheduled so that they get done and don’t interfere with other projects that might be on a tight deadline. And then there is inventory management, making sure you have the materials you need on hand when you need them. There is also the occasional rescheduling that occurs if equipment breaks down or someone is out sick. A simplified workflow based on the above is shown in Figure 2.
|
Let's say our print shop has seven people working there. The owner manages the overall operation, and an administrator logs in work orders, notes the location of files that will be used on those orders, and does the final checkout of work, shipping, and billing. The remaining five people—staff, although everyone is at the same organizational level—take care of the actual production work; everyone is cross-trained, but some are more adept on some tasks than others.
Imagine you worked in this shop; how might your day go if you were one of the staff? The administrator will have prioritized the work depending on urgency and grouping similar work orders (or partial orders if there is request for multiple services) together. This is just a matter of efficiency: if you are using a particular piece of equipment and it has to be set up, calibrated, and cleaned when finished, you may as well make the most of that effort and run as many similar jobs as you can. Tracking down copies of work orders is an issue if someone is already working part of the order as there is only one copy so that notes and comments don’t get lost. Each staff member has a notebook to keep track of work, any settings used on equipment, and comments about how the work progressed. These notebook entries are important and useful in case questions come up about a job, how it was run, and if any issues were encountered. As one set of jobs is completed, you move on to the next set. Inventory has to be checked to make sure that the needed materials are on-hand or ordered; if something is missing work has to be rescheduled. The workflow is a continual, organized mix of tasks, with people scheduling time on equipment as needed.
You can begin to appreciate how difficult the manual, paper-based workflow in a shop like that is to manage, particularly when it depends upon people communicating clearly. It is the same workflow as any service-oriented business, from a florist to a repair shop. What differs is the size of the organization, the complexity of the work, and the education needed to perform the required tasks.
Now let's get back to the service laboratory. The print shop workflow is much like the structural workflow of such a laboratory. In the end, it’s the nature of the tasks; the complexity of equipment, instrumentation, and electronic systems used; and the education needed to carry out the work that sets the service laboratory apart from other service operations. However, there is one other, critical aspect that sets it apart: most service labs have to meet federal or industry regulations (e.g., the ISO 9000 family of standards) for their operations.
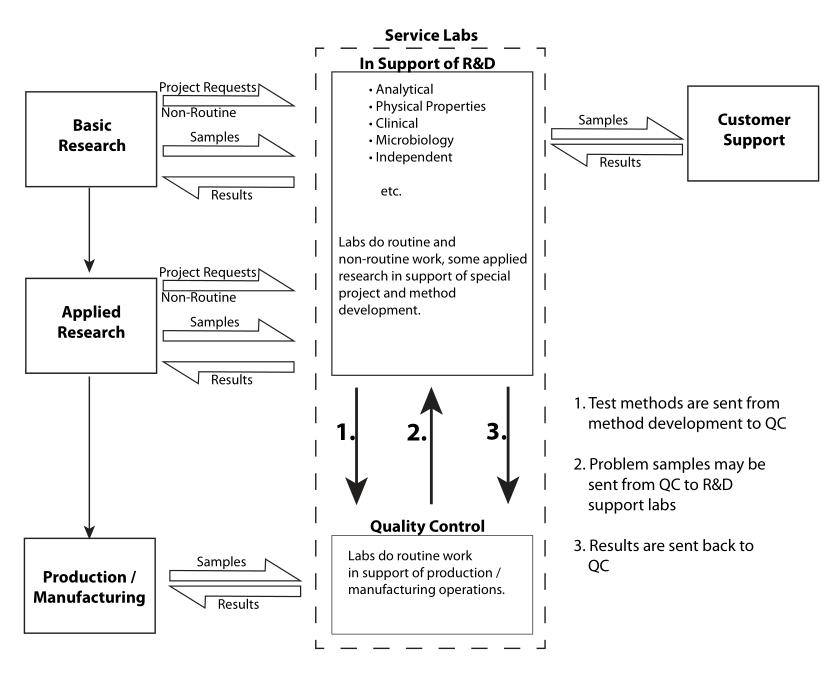
As noted earlier, there are many different types of service laboratories. The basic workflow is the same (see Figure 3 for one perspective on the commonalities of research and service laboratories), but the nature of the testing separates one from another. A water testing lab uses different test procedures than a toxicology lab does, or a clinical lab. Those working in different types of labs have to learn how to run different tests, and they also have to learn about the materials they work with. After all, people's observations about the material tested will differ depending upon how much experience they have with different kinds of materials.
|
While workflows vary between research and service labs, there is one consistent factor that cuts across both: record keeping.
The laboratory notebook
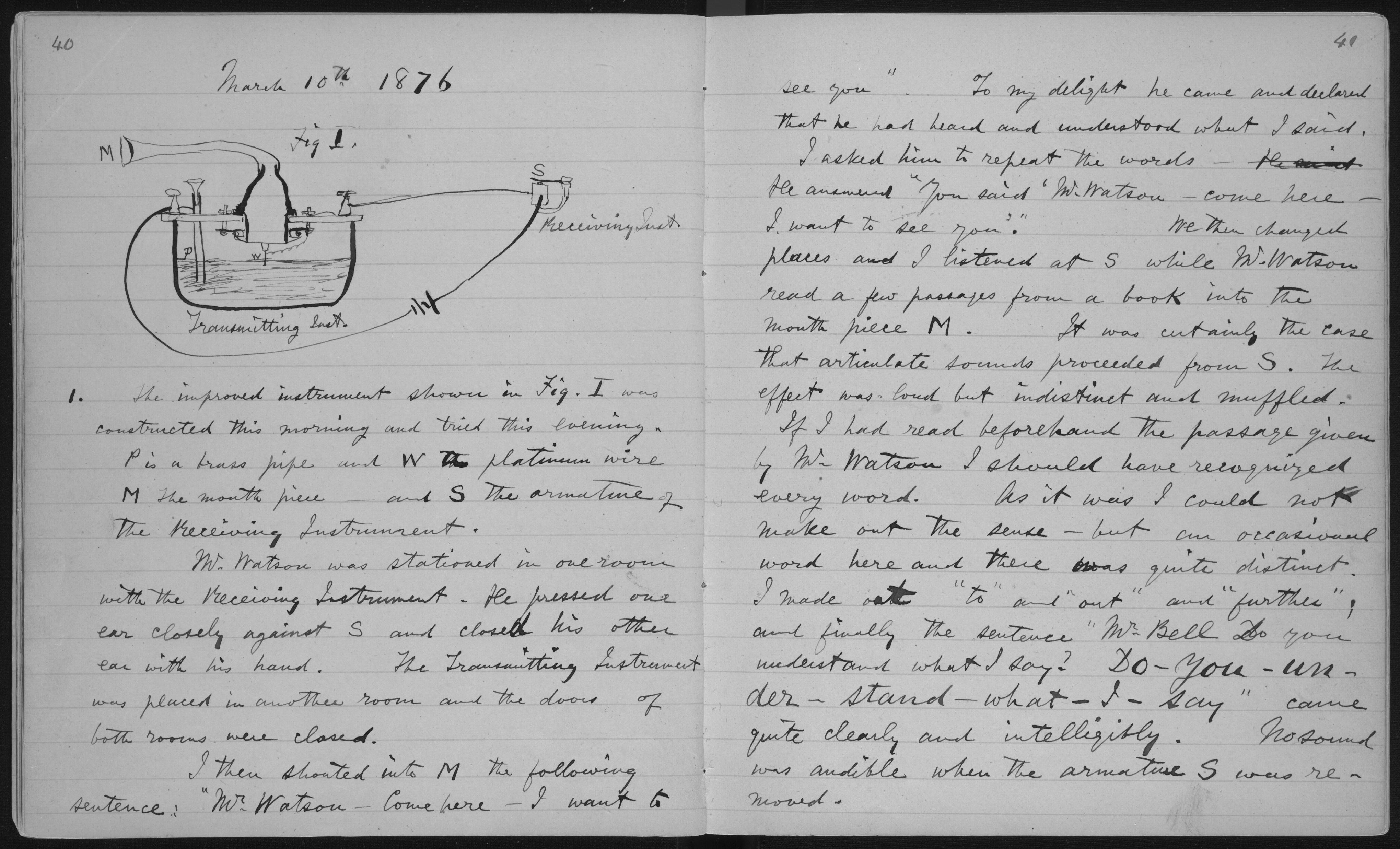
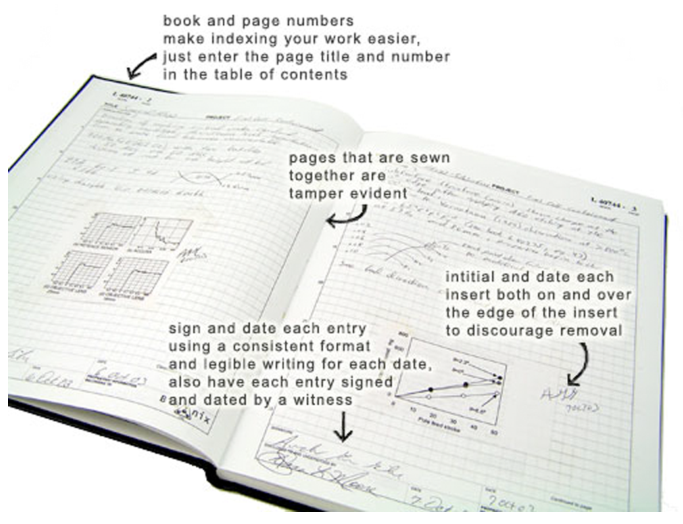
The laboratory notebook has been a fixture in scientific work for centuries. The laboratory notebook is essentially a diary and can contain text, drawings, pasted photos, illustrations, charts, and so on. Historically, at least until the mid-1970s, it was a paper document that has evolved as legal and regulatory considerations have developed. Figure 4 shows part of Dr. Alexander Graham Bell’s notebook.
|
The format of today’s paper notebooks has changed somewhat, and the process of using it has become more rigorous. Take for example Scientific Bindery Productions, a modern manufacturer of professional laboratory notebooks. The description for their duplicate lined notebook includes the following elements[1]:
- table of contents
- instructions page, for how to use the notebook and address patent protection
- headers and footers, with legally defensible language
- headers that include title, project number, and book number fields, as well as a "work continued from page" section
- footers that include signature, date, disclosed to, and understood by fields, as well as a "work continued to page" section
The details of some of these points are called out in Figure 5, courtesy of Dr. Raquel Cumeras.
|
Over the years, several guidelines have been published about the use of laboratory notebooks. Examples include:
- Good manufacturing practice (GMP) and good laboratory practice (GLP) recordkeeping, from David West, St. Louis Community College, Center for Plant and Life Sciences[2]
- NIH scientific recordkeeping guidelines, from the National Institutes of Health[3]
- General laboratory notebook guidelines, from Science editor Elisabeth Pain[4]
A Google search of "guidelines for maintaining laboratory notebooks" or something similar will provide more examples, including those developed by leading universities.
At this point, you’re probably wondering why we’re spending so much time on this. The point: good record keeping is the foundation for documenting scientific work regardless of the media, be it paper or electronic. Yes, the laboratory notebook has an electronic equivalent: the electronic laboratory notebook (ELN). These ELNs and other laboratory informatics systems have to support everything paper systems do or they will fail in ensuring the integrity of documented work.
Using laboratory records
Laboratory records, whether in laboratory notebooks or some other format, can be acted upon in many ways. Laboratory personnel interact with them by:
- recording observations, results, instrument data output, photos, and charts
- describing research processes, goals, and results
- ensuring the authenticity of laboratory work
- planning and collaborating on experiments
- extracting information for reporting
- backing up data
- querying data
- sharing data
- publishing data
- archiving and retrieving data
- securing data
Everything on that list can be done with paper records; however, those activities are easier, faster, and less error prone with electronic systems. Paper records aren’t going away anytime soon, for example when needing to record comments and information that may not have been provided for in electronic systems. This is particularly true as a project team expands from one person to more people. However, the need to have shared access to information becomes a limiting factor in productivity when we rely on paper-based systems. Paper-based systems also depend upon the proximity of people working together, something that became problematic during the COVID-19 pandemic. Social distancing requirements made sharing paper-based notebook pages more challenging, requiring scanning and emailing. This was perhaps feasible for small amounts of physical materials, but less so for large projects with significant paper-based records.
That brings up another important point concerning ownership: whose data is it? When people are handed a notebook, they are told “this is your notebook, a place to write down your work and observations, and you are responsible for it.” Depending upon how employment or consulting contracts are written, the content that goes into the notebook belongs to whoever is paying for the work. When I worked in a lab, the notebook I used as mine was referenced by others as “your notebook” (it even had my name on it) even though it wasn’t mine but rather the company’s property. Yet when it was filled, they took possession of it and archived it. This concept of ownership has become a stumbling block in some organizations when they decide to install an ELN or laboratory information management system (LIMS), particularly if there are people who have been working there for a long time and have ingrained behaviors. Those people become concerned that someone is going to see their work in an incomplete state before they’ve reviewed and completed it. It’s their work and they don’t want anyone to look at it until it’s done. While the true owners of the work have always had that right, they may not have exercised it, respecting people’s privacy until the work is complete. If you’re considering an informatics system, does it address that concern about ownership?
Bringing informatics into the lab
So far this guide has hinted at the implications of adding laboratory informatics systems into the laboratory, but now it's time to discuss it further. Deciding how and when to bring such systems into the lab depends on a number of factors.
1. What is the lab's budget? If an informatics implementation can't be properly funded, don’t start that implementation until it can be. The cost of a laptop computer is trivial compared to the total cost of implementation.
2. Do we have in-house access to educated and experienced IT support? That staff should understand that laboratory operations are not just another PC- or Microsoft-dominated arena, but rather an environment which has needs for informatics solutions beyond the typical office solutions. For example, laboratory instruments need to be connected to computers, and the resulting data stores should ideally be integrated to make the data more actionable.
3. Are laboratory staff ready to use the technologies and take responsibility for the things that go with it? Staff must be trained in more than how to operate an instrument. Can they back up data? Do they understand the security and data privacy risks associated with handling the data?
4. Are organizational policies flexible enough to allow practical use of the technology while still keeping security and data privacy risks in mind? The organization should have some sort of network access both internally and externally. Remote access should be possible, particularly given the circumstances surrounding pandemics and the like. A balanced policy on taking an organizational laptop out of the laboratory should be in place. Policies on cameras should also be reasonable, allowing researchers to capture images of samples and specimens for their notebooks. If organizational policies are too restrictive, the technology's usefulness is largely overshadowed.
5. What are the lab's space constraints? The size of a lab and the experiments it must conduct can affect the choice of informatics tools.
6. What is the nature of the lab's operations? Is it a research lab, service lab, or a combination of both? If you are in a service lab situation, bringing in informatics support as early as possible is essential to your workflow and sanity. You want to minimize having to deal with two separate processes and procedures: the old way we did it (paper-based) and the informatics-supported way.
7. Is your lab’s operation governed by external regulatory requirements? If it’s going to be in the future, you may as well start as though it currently is. Note that everything should be validated, regardless of whether or not the lab is subject regulations. Validation isn't done to satisfy regulators but rather to prove that a process works properly. If you don’t have that proof, what’s the point of using that process? Do you really want to trust what a process produces without proof that it works?
Most of the points above are easily understood, but let's go into further detail. Let's start by looking at a simple case of you and your project, where you are the sole contributor, without any need for regulatory oversight. Your primary need is to record your planning notes, observations, results, etc. There are tools within the world of computer software to help with this, most notably the word processor. If you have access to one of those, you probably also have access to spreadsheets and other applications that can make your efforts far easier than working with paper. If you search Google for “word processors as lab notebooks” you will find a number of references, including Dr. Martin Engel's guide to using Microsoft OneNote as an ELN[5] and Labforward's 2020 ELN selection guide.[6]
However, simply switching from paper to electronic doesn't mean you're done. There's more to consider, like developing backup policies, addressing witness review, connecting to instruments, and addressing the effects of team expansion, including expanding to more comprehensive purpose-built informatics solutions.
Backup strategy
You have the electronic documentation tools and the skills to use them, but what else do you need? A backup strategy is imperative. Imagine a scenario where you are using a desktop computer, laptop, or tablet to do your work and it has one copy of the document you’ve been working on for weeks. You press the power button one morning and nothing happens. However, you are not (completely) worried or panicked but rather largely calm because:
- you have a local backup on removable media (e.g., flash drive, disk), several instances, in fact, that were backed up at least daily, with backups containing everything on the system you were using (you may have a separate backup of your project);
- you have a remote backup on your organization's servers (perhaps on a virtual machine);
- you have a cloud-based backup of at least your project files, and as much of the system files that the cloud storage permits (depending on bandwidth and cost), all secured with two-factor authentication; and
- depending on the operating system you are using, you may have built-in backup-and-recover abilities, e.g. as with Mac OS X's "Time Machine" functionality.
You've done these things because you've asked yourself "how much is my work worth to me and my organization?"
Witness review or sign-off
The need for a witness review or sign-off can occur for several reasons, including a potential patent filing or proof that the work was done and data recorded properly, on a certain date, in case it is challenged. One of the ramifications is that you have to identify a second person to be that witness (though this would also be the case if you were using a paper notebook).
A second issue is that you would have to format the pages of your word processor document (using templates) so as to emulate a signature block and page numbering structure that meets the requirements noted earlier for paper notebooks. You also have to provide a means for either physical (printouts) or electronic signatures (e.g., Adobe Acrobat and other applications provide a useful template that could be used as a lab notebook in this case, doing a cut-and-paste from a Word file). You would also have to ensure that once dated and signed, no edits could be made to that material. If you choose the printed route, then you’re back to paper management. One possibility for dealing with that is to scan the signed pages and upload them to a secure server using the file server's date and time stamp system, or to a document management system to demonstrate that documents haven’t been changed.
There is another possibility for time-stamping printed material that is scanned with a high-quality scanner. The concept of machine identification code or "tracking dots" allows a set of barely perceptible, forensically traceable colored dots to be printed onto pages, with the arrangement of the dots providing information that identifies the printer (by serial number), as well as the date and time a page was printed. Recent research has demonstrated ways to decode these dots for analysis, particularly as part of printer forensics.[7][8]
Instrument-computer integration
Using a computer to host a laboratory notebook raises another concern: how can lab instruments or separate instrument data systems connect to automatically transmit data and information to the computer? Controlled data collection will require software beyond a simple word processor, though many of today's ELN vendors provide integration components with their solutions. This connection, possibly using a spreadsheet import, will greatly improve laboratory productivity and the reliability of data transfers.
Expanding the research team
Increasing the size of your project by adding more people will have a significant impact on the complexity of using a more electronic laboratory workflow. However, while the basic issues of sharing, collaboration, etc. are not any different than they would be if paper-based notebooks were in use, the electronic solutions to handling those issues are much more useful. At the core of an expanded laboratory operation is getting personnel to agree on how they are going to communicate data and information, how they are going to collaborate using that data and information, and how they will make that agreement sustainable. There is going to be some small allowance for individual approaches to laboratory activities, but critical matters such as data organization and management need to be strictly adhered to. As such, there are several issues to be mindful of.
1. How will data and information be organized and compiled? If multiple people are contributing to a project, the results of their work need to be organized in one place so that the status of the work can be ascertained, missing data can be identified, and material is easier to work with. As a project's direction evolves, the formatting and content may change, potentially requiring a complete overhaul of the data structure. That is just a consequence of the way research programs progress.
2. How will data and information be updated, versioned, and retained? If your lab works with paper files, this isn't so difficult. The lab may have one printed file detailing experimental methods, and when that method file gets updated, the old printed document is removed and the new one added. In labs where each person has their own individual method file, the process would be repeated for each person. As such, there's no confusion as to the current version; methods would have revision histories so that you would know why they were changed. In cases where those methods are kept in electronic files, more attention has to be paid to ensuring that old method files are archived, and that everyone is working with the same version. This means clear communications procedures are essential. Additionally, the name of the file should have the current revision information; don’t rely on the computer's creation or modification dates for files.
3. How is access to common files controlled? Having people edit or add to common files without careful lockout controls is dangerous. If two people open the same document at the same time and make changes, the one who saves the file last can overwrite any changes the other individual has made. You need a document management system that prevents this; if a file is checked out by one person, others may read it but not write to it until the first person is done. Old versions of files should be archived but not deleted so that a detailed revision history is maintained.
If an organization grows too large for a consensus-based cooperation of people using office suite products, it will be time to transition to a more purpose-built solution like a multiuser ELN that is capable of allowing multiple people to contribute to a project simultaneously, providing a common organization for data and information, and allowing users to either import data from an instrument or have a direct connection between the ELN and the instrument data system (or through the use of scientific data management system [SDMS]). But how large is too large? It may be the point when personnel become frustrated with waiting for access to documents, or when processes just don’t seem to move as smoothly as they used to. While there is a significant cost factor in implementing an ELN, it should be done sooner rather than later so your lab can benefit from the organizational structure that an ELN provides and reduce the amount of effort it will take to import data and information into the structure. Ideally you are better off if you can start your work with an ELN once the initial project startup work is taken care of.
ELNs afford a number of additional benefits, including but not limited to access to:
- vendor databases for ordering supplies and managing inventory,
- chemical structure databases,
- reaction databases, and
- external libraries.
ELNs are appropriate for both basic research and applied research, including work in testing labs where method development and special project work is being done. The prior bulleted list might give you the impression that ELN are oriented toward chemistry; however, that is a reflection of my experience not the industry. The ELN is used in many other industries. Several examples of ELNs past and present, covering many industries, are listed below.
- LabTech Notebook was released in 1986 and discontinued in 2004, and it was designed to provide communications between computers and laboratory instruments that used RS-232 serial communications. This ELN was applicable to a variety of industries and disciplines.
- SmartLab from Velquest was released in the early 2000s and was the first commercial product to carry the "electronic laboratory notebook" identifier. It was designed as a platform to encode and guide people conducting lab procedures in GLP/GMP/GALP environments. Now owned by Dassault Systèmes and rebranded as a laboratory execution system (LES) as part of the BIOVIA product line, the solution's same conceptual functionality has since been incorporated into LIMS and ELNs that fit the more current expectation for an ELN.
- Wolfram Research has as series of notebook products geared toward mathematics.
There is a growing list of ELN systems in a variety of disciplines. Given that the scope of activities an ELN can cover is fairly broad, you have to be careful to define your needs before uttering the words “we’re going to implement an ELN.” We’ll address that point later.
The organization of an ELN application is flexible. The layout is user-defined and can contain queryable text, figures, graphics, and fields for instrument-generated data. Because the ELN is inherently flexible and there usually isn’t any quick-start structure, you have to know what you are looking for in a product and how you want to use it. This requires quality due-dilligence research. “If only I had known” are among the saddest words after product selection.
Some organizations have chosen to develop their own ELNs. That is something that should be undertaken with fear and trepidation. Part of the original justification for this route is typically based on the belief that you have special needs that can't be met otherwise. Another concern may be that you don’t want to tie yourself to a vendor that may go out of business. Those points are more a matter of product selection criteria than a justification for a software development project. If you do choose to go with an internal or even a contracted development route, you will potentially have to live with a product that has just one customer (unless the contractor decides to productize it, which is an entirely different discussion). You will also be saddled with the support of that product for the rest of its functional life. And that doesn’t even get into the "who" of software product management and development, the “when will it be done,” and the inevitable scope creep (i.e., the change and expansions of development requirements). In the initial stages of research projects, the organization of data is subject to change as needs change. This is often at the behest of “unstructured” data and the need to manage it. (Note that it isn’t that the data is truly unstructured, it’s just in a variable structure until the project finds its direction.) This can lead to frustration in setting up a commercial ELN system, let alone designing one from scratch. The next section will look at an organization that is more highly structured.
Meeting the needs of the testing laboratory
The earlier print shop description will give you an idea of the workflow of a testing or service lab, for example a quality control (QC) lab. In a manually managed QC or research laboratory, operations can become overwhelming quickly. Imagine a study of 40 samples[b] with multiple tests per sample, each of which has to be individually cataloged. Actually I don’t have to imagine it; it happened frequently in our lab, which supported several research groups. When one of these large sample sets shows up—and sometimes more than one—you don't talk to the person doing the sample logging for fear of disrupting their required concentration. You can get them coffee, but no talking.
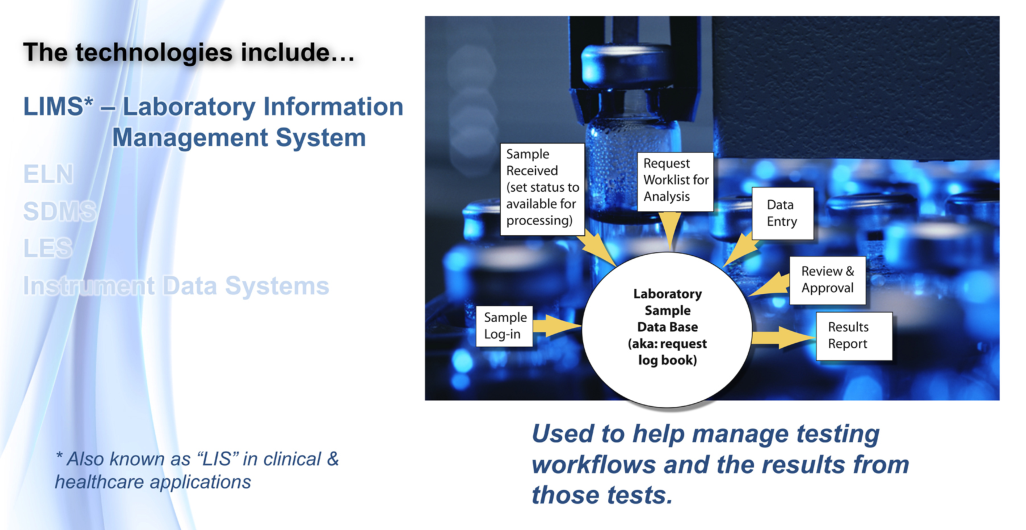
With a LIMS, this isn't so much an issue. You can log in one sample or a hundred, simply by telling it the starting sample ID, how many samples, and what tests should be scheduled. The LIMS then organizes everything and prints the labels for each sample. With some systems, the requestor of a test can even log them in from a web portal, and the central LIMS automatically updates when the samples actually arrive in the lab.
A LIMS makes life easier for laboratories in a number of other ways as well. Want to find a list of samples that are pending a particular test? A quality LIMS can readily display that information, including the sample numbers, priorities, and current locations, with no need to manually check work request sheets. Does a third party want to find out the status of one or more of their in-process samples? Role-based access management means a third party can receive limited access to view that status, without seeing anyone else's sensitive data. What about verifying and approving results? The LIMS can provide some level of results checking, with final verification and approval by lab management. When approved, the reports for each set of requests can be printed, emailed, or stored for portal access. And what about integrating data and systems? The LIMS can be connected to an instrument data system (IDS). Depending on the sophistication of that system, the LIMS can generate a worklist of samples that needs to be processed by that device, with the list downloaded to the IDS. When the work is completed, the results can be uploaded directly to the LIMS. This type of system interaction is one of the places where significant productivity gains can be had.
The key attributes of a LIMS are shown in Figure 6.
|
These are just the highlights of what a LIMS can do. Why is a LIMS more effective out-of-the-box than an ELN? The operational behavior of testing labs is essentially the same across industries and disciplines, and as a result vendors have a more predictable and stable base of customers and user requirements to work against than an ELN. It’s a bit like comparing home building contractors. Some have a basic structural architecture that they can modify to meet the needs of a broad market. Others do completely custom homes from the basement up, each of which is unique. The economies of scale and a broader, more predictable customer base show up in products that are easier to work with and adapt both for the primary vendor and those providing add-ons. Those LIMS add-ons include specialized facilities for different industries, including enology and viticulture analysis, water treatment, mineral analysis, cannabis testing, and clinical diagnostics (though the system used in the clinical setting is typically referred to as a laboratory information system or LIS). Regardless, and as is the case with ELNs, you want to install a LIMS as soon as you are able to avoid issues surrounding differing workflows based on pre- and post-implementation.
Other laboratory informatics systems
We've mentioned a few other systems in passing, but here we'll provide a brief overview of a few of them.
Scientific data management system (SDMS)
The SDMS helps laboratories better solve the problem of dealing with a large number of data files that are being generated, basically by acting as a giant file cabinet that LIMS, ELN, and IDS can connect to. For example, you may not want to put large datasets, spectra, etc. in a LIMS or ELN, you but still have to reference those large files within other internal LIMS and ELN files. This is where the SDMS steps in, storing those large files in the "file cabinet" while maintaining references and metadata that are usable by other informatics systems.
Laboratory execution system (LES)
You may not know about them, but there are lesser-known systems called LES that are designed to ensure testing protocols are executed properly and that all the data that is necessary is properly recorded. The initial incarnation arose from the previously discussed SmartLab[c], a stand-alone product that would guide an analyst through the steps of an analysis, recording each detail of the work in a file that regulatory agencies could inspect to ensure that work was done properly. It found a ready market in any lab that needed to meet GLP/GMP requirements. The functionality needed to create the same capability can be found in some LIMS and ELNs, but programming is required.
Instrument data system (IDS)
Any laboratory instrument popular in the marketplace has had a computer either attached to it or built into it as a package. That’s what an IDS is. It provides automated control over an instrument, collecting and analyzing the data produced by the measuring components. Depending on the sophistication of the vendor, and the demands of the marketplace, the connections between the IDS and another laboratory informatics solution may range from user-programmable interfaces (via a network, USB, serial, or digital I/O connection) to built-in communications systems that are almost plug-and-play. The latter are most commonly found in the clinical chemistry market, where a great deal of attention has been paid to integration and systems communication via Health Level 7 (HL7) and related protocols. (The details, however, are beyond the scope of this document.)
Planning for laboratory informatics
There are two key requirements to the successful implementation of informatics products: education and planning.
Education
As far as education goes, the webinar series noted in Figure 6 is a good tool, as are documents provided by technical standards body ASTM International. ASTM documents that may be of value to you include:
- ASTM E1578-18 Standard Guide for Laboratory Informatics[9]
- ASTM E1578-13 Standard Guide for Laboratory Informatics[10] (contains some information not found in 2018 version)
- ASTM E1947-98(2014) Standard Specification for Analytical Data Interchange Protocol for Chromatographic Data[11]
- ASTM D8244-21 Standard Guide for Analytical Laboratory Operations Supporting the Cannabis/Hemp Industry[12]
- If you search the ASTM website for "LIMS" or "informatics," you'll be surprised by the amount of other material that shows up.
Other sources of educational material include:
- The Tutorial section of LIMSforum.com[13]
- Laboratory Information Systems Project Management: A Guidebook for International Implementations, by the APHL
- Laboratory Informatics Guide 2021, by Scientific Computing World[14]
- Lab Manager magazine, published by LabX Media Group[15]
- Computerized Systems in the Modern Laboratory: A Practical Guide, by Joe Liscouski[16]
- Any vendor with an informatics product will send you a nearly endless stream of material.
Ultimately, however, the responsibility for informatics implementation projects doesn't exist solely on the shoulders of laboratory personnel. Everyone connected with a laboratory implementing informatics solutions should have some level of awareness regarding laboratory informatics, including upper management. However, the level of knowledge required may vary slightly depending on the role. For example, laboratory personnel should be fully educated on common laboratory technologies at a minimum. They should also understand why an informatics project is being considered, what the scope of the implementation will be, and what their role will be in the implementation. Upper management should remember to ask laboratory personnel for input on the project, including the topic of product requirements, in order for personnel to feel like they are part of the process, not simply an observer or "something that's happening to them." Finally, laboratory personnel should understand how their jobs are going to change once the implementation is complete. This needs to be addressed very early in project discussions; it is a matter of change, and change scares people, particularly if it affects their job and income. This is not a “we’ll deal with that later” point. Don’t start the discussion until you figure this out. Things may change, but people want security.
Of course, any information technology (IT) personnel will also be involved, requiring significant knowledge about not only networking and software installations but also systems integrations. IT personnel need to understand their role in the implementation project, which can include support, project management, evaluating the vendor support capabilities, and more. They should also be fully aware of and understand the technologies the organization is considering for implementation, and they will be a vital part of the project planning and vendor selection process. IT personnel also will be interested in questions about any enterprise resource planning (ERP) aspects, which may raise issues of "build or buy." The organization needs to be prepared to both address these concerns and gain IT personnel as strong supporters of the project.
Finally, upper management—those who are going to approve the project and provide funding—need to be educated enough to understand the benefits and risks of the proposed implementation, including the likely time scale for the work. Upper management will need to be active in the project in at least two critical junctures, plus at specific milestones as needed. The first time upper management will need informed participation will be during initial project planning. They will help the organization lay out the issues that need to be addressed, the scope of options that will be investigated, and how the organization is going to proceed. They may pose questions such as “can we use existing system to solve the problem,” particularly if there already has been an investment in an ERP solution such as SAP. Such technology questions will also be of interest to IT personnel since they have an investment in those systems. The second time upper management needs to undoubtedly be involved is when the actual project proposal is finished and is ready to be pitched. They will ask need to ask questions about the reasoning behind the choices made, why current systems are insufficient, what kind of investment the project will require, how the implementation will be scheduled, and how the roll-out would be planned. Understanding the answers to these and other questions will be difficult if upper management doesn’t understand the technology, the issues, the options, and the benefits of the proposed laboratory informatics project.
If the world of informatics is new to any or all these stakeholders, the organization must consider getting outside support (not from a vendor) to guide the organization through the process of evaluating the lab's operations, scoping out an overall implementation program, dividing it into milestones, setting priorities, and developing user requirements.
Planning
The whole point of project planning is to get your organization from the starting point to the end goal, preferably using the most effective path. So, where is the organization going, and why? Everything pretty much boils down to those two questions. Once those questions are answered, more will arise concerning how the organization is actually going to get to the project's end goal.
Initial planning will look at the lab from a standpoint of the minimum number of computers required to do the work. In some cases, perhaps just those computational systems built into instruments will be sufficient. Whatever the decision, that’s the planning baseline. Then consider what kind of lab it is: a research lab, a service lab, or a blend? That helps direct concentration on potential solutions; however, be sure not to completely eliminate other options just yet. For example, if your lab is a QC lab, it's probably a service lab in need of a LIMS, but even then there are still options to evaluate.
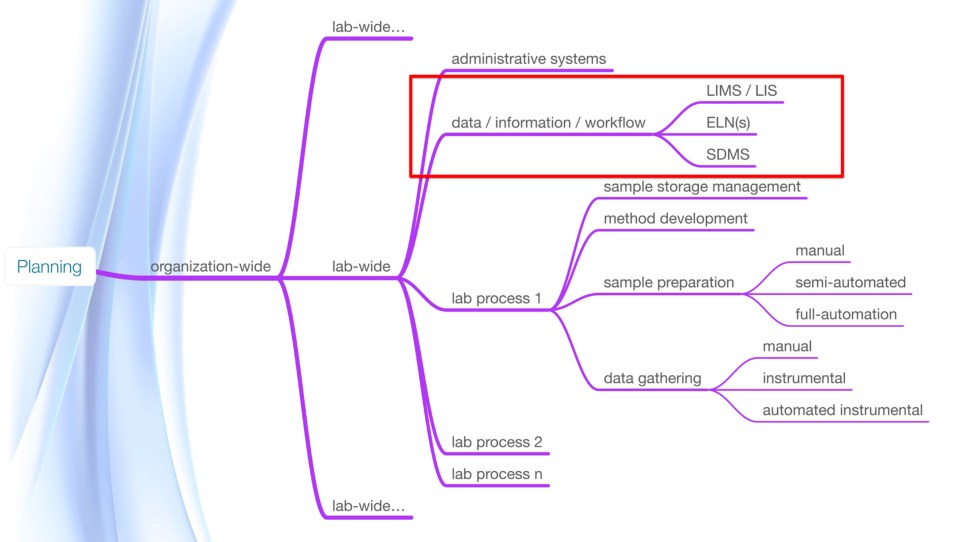
From there, the organization must also think in terms of where that baseline lab is going to be in five years; it’s an arbitrary number but a starting point. Why that far out? It will take a year or two to plan, implement, validate, and educate people to work with the new systems and for the lab to change its behavior and settle down to a new mode of working. The organization should also consider what other changes are likely to take place. What new equipment, procedures, and management changes can be anticipated? Ideally any implemented informatics system will be in place and stable for a few years before people start asking for significant changes; minor ones will likely happen early on. Any hint of “we didn’t plan for that” will be viewed as poor leadership. Figure 7 shows some of the key points you need to look at during planning.
|
Another factor that needs to be considered is that the considerations shown in Figure 7 can be repeated for each lab in the organization. The project planning team also needs to consider how current laboratory workflow impacts other labs. Are there synergistic effects that can be gained by broadening the scope of what the lab is doing?
Why projects fail
We shouldn't finish the planning section of this guide without discussing why laboratory informatics implementation projects can and do fail. These types of projects are large software projects, and delays, cost over-runs, and failures are common (but hopefully not for your organization's project). Just Google "why IT projects fail" and read through some anecdotes. The following are some common reasons informatics implementation projects fail.
- Insufficient budgeting: Projects can run short of funding, requiring an awkward meeting with management asking for additional funding (without a project change to account for it), inevitably showing a lack of foresight and planning. Build in a large contingency fund because the unexpected happens. If you’d like some education on the topic, watch a few episodes of any home upgrade project on HGTV, in particular Love It or List It.[d]
- Insufficient management support: If sufficient communication isn’t made with management, problems may arise. For example, project delays are a fact of life. Keep clear communications with upper management so that they, and everyone else on the project, know what is going on. Miscommunication or lack of communication of other aspects of the project may inevitably doom the project.
- Poor understanding of the scope and nature of the project: This is an educational issue, and a lack of education for all involved parties is almost a guarantee of failure. If you need help, bring in an independent consultant who can lend confidence to the project and its management.
- Lack of quality or attention to detail: “There is always time to do it over, but not enough to do it right” is a common complaint in engineering projects. If you hear it on your organization's project, you are in trouble. Basically the complaint is that project members are cutting corners and not doing things properly, and in a way that is not supportable. This never ends well; sometimes not quickly, but in the long run it leads to problems.
- Poor or unrealistic timelines: You may as well face reality from the start: an aggressive timeline just leads to problems (see bullet above). Timelines expand, but they almost never get shorter. If the project team is always rushing, something will most certainly get missed, causing problems later down the road.
- Poor project management: Well-managed projects are obvious, and so are poorly managed ones. Just watch the people working, their demeanor, and their attitude about the work; it will tell you all you need to know. Well-managed projects may not always run smoothly, but they make consistent progress. Poorly managed projects cause you to make excuses.
Closing
This guide is like the directory signs in shopping malls: they tell you where you are and what shops and restaurants in the facility to consider. Once you figure out what you are looking for, you can find your way there. Hopefully in reading this you’ve formed an idea of what you want to look at and what your path to finding it is.
Footnotes
- ↑ According to Greek mythology (from the E2BN Myths page): "Long long ago, when Queen Athena (Zeus's daughter) was born, Zeus blessed her with two boons for when she came of age. After almost 15 years, Athena was told to think up two things to ask for ... 1) To have a city in Greece named after her (Athens) [and] 2) To have all the people of the world see her face every day of the year (what you are seeing are only her eyes). Thus, the sky is blue, just like the color of Athena's eyes..."
- ↑ In some life science drug screening studies, the number can be far higher, which is where robotics and automation becomes important.
- ↑ A name that has been used by conference organizers after the product was sold and renamed. If you do a Google search on “SmartLab,” you may be surprised at what turns up.
- ↑ Some season highlights can be found on the HGTV website.
About the author
Initially educated as a chemist, author Joe Liscouski (joe dot liscouski at gmail dot com) is an experienced laboratory automation/computing professional with over forty years of experience in the field, including the design and development of automation systems (both custom and commercial systems), LIMS, robotics and data interchange standards. He also consults on the use of computing in laboratory work. He has held symposia on validation and presented technical material and short courses on laboratory automation and computing in the U.S., Europe, and Japan. He has worked/consulted in pharmaceutical, biotech, polymer, medical, and government laboratories. His current work centers on working with companies to establish planning programs for lab systems, developing effective support groups, and helping people with the application of automation and information technologies in research and quality control environments.
References
- ↑ "Duplicate Lined Notebook". Scientific Bindery Productions, Inc. https://scientificbindery.com/products/duplicate-lined-notebook/. Retrieved 12 May 2021.
- ↑ West, D. (26 April 2024). "GMP/GLP Recordkeeping". St. Louis Community College, Center for Plant and Life Sciences. https://users.stlcc.edu/departments/fvbio/Lab_Practices_GLP_STLCC.htm. Retrieved 12 May 2021.
- ↑ National Institutes of Health (December 2008). "Guidelines for Scientific Record Keeping in the Intramural Research Program at the NIH" (PDF). Office of the Director. https://oir.nih.gov/sites/default/files/uploads/sourcebook/documents/ethical_conduct/guidelines-scientific_recordkeeping.pdf. Retrieved 12 May 2021.
- ↑ Pain, E. (3 September 2019). "How to keep a lab notebook". Science. doi:10.1126/science.caredit.aaz3678. https://www.sciencemag.org/careers/2019/09/how-keep-lab-notebook. Retrieved 12 May 2021.
- ↑ Engel, M. (December 2015). "Blog: How to use onenote as your electronic lab book". MartinEngel.net. http://martinengel.net/2015/12/how-to-use-onenote-as-your-electronic-notebook/. Retrieved 12 May 2021.
- ↑ Bungers, S. (2020). "The Electronic Lab Notebook in 2020: A comprehensive guide". Labforward GmbH. https://www.labfolder.com/electronic-lab-notebook-eln-research-guide. Retrieved 12 May 2021.
- ↑ Richter, T.; Escher, S.; Schönfeld, D. et al. (2018). "Forensic Analysis and Anonymisation of Printed Documents". Proceedings of the 6th ACM Workshop on Information Hiding and Multimedia Security: 127–38. doi:10.1145/3206004.3206019.
- ↑ Baraniuk, C. (7 June 2017). "Why printers add secret tracking dots". BBC Future. Archived from the original on 02 November 2019. https://web.archive.org/web/20191102031255/https://www.bbc.com/future/article/20170607-why-printers-add-secret-tracking-dots. Retrieved 13 May 2021.
- ↑ "ASTM E1578-18 Standard Guide for Laboratory Informatics". ASTM International. 2018. doi:10.1520/E1578-18. https://www.astm.org/Standards/E1578.htm. Retrieved 13 May 2021.
- ↑ "ASTM E1578-13 Standard Guide for Laboratory Informatics". ASTM International. 2013. doi:10.1520/E1578-13. https://www.astm.org/DATABASE.CART/HISTORICAL/E1578-13.htm. Retrieved 13 May 2021.
- ↑ "ASTM E1947-98(2014) Standard Specification for Analytical Data Interchange Protocol for Chromatographic Data". ASTM International. 2014. doi:10.1520/E1947-98R14. https://www.astm.org/Standards/E1947.htm. Retrieved 13 May 2021.
- ↑ "ASTM D8244-21 Standard Guide for Analytical Laboratory Operations Supporting the Cannabis/Hemp Industry". ASTM International. 2021. doi:10.1520/D8244-21. https://www.astm.org/Standards/D8244.htm. Retrieved 13 May 2021.
- ↑ "Tutorials". LIMSforum. LabLynx, Inc. https://www.limsforum.com/category/education/tutorials-education/. Retrieved 13 May 2021.
- ↑ "Laboratory Informatics Guide 2021". Scientific Computing World. 2021. https://www.scientific-computing.com/issue/laboratory-informatics-guide-2021. Retrieved 13 May 2021.
- ↑ "Lab Manager". LabX Media Group. 2021. https://www.labmanager.com/magazine. Retrieved 13 May 2021.
- ↑ Liscouski, J.G. (2015). Computerized Systems in the Modern Laboratory: A Practical Guide. DHI Publishing. pp. 432. ISBN 9781933722863. https://www.dhibooks.com/computerized-systems-in-the-modern-laboratory-a-practical-guide.