LII:The Practical Guide to the U.S. Physician Office Laboratory
The Practical Guide to the U.S. Physician Office Laboratory
Written by: Rebecca A. Fein, M.S.A.H.I., M.B.A.
Edited by: Shawn Douglas
Introduction
This guide intends to give the reader practical information on the physician office laboratory (POL), assisting the reader with the decision-making processes related to becoming affiliated with a POL.
This guide provides a discussion on the history and trends related to the POL market, as well as testing considerations, staffing requirements, regulatory issues, related technology, and economic considerations.
No recommendations are made, though appropriate best practices are mentioned.
What is a physician office laboratory?
The definition of a physician office laboratory varies from state to state. Some states define the POL by the actual number of physicians in the practice, and others do not. When setting up a POL, the proprietor should consult their individual state regulatory body to ensure full compliance.
For the purpose of this paper, the definition provided by the State of New York is used, as New York regulations are strict, and using a stringent guideline for the rest of this guide is preferred:
In order to qualify as a physician office laboratory (POL), individual health care providers must operate the practice or be part of a legally constituted, independently owned, and managed partnership or group practice. Laboratories that are owned, managed and/or operated by managed care organizations, hospitals or consulting firms do not qualify for the POL exception and should apply for a clinical laboratory permit through the Clinical Laboratory Evaluation Program (New York State Department of Health, Wadsworth Center, 2014).
Based on the New York definition, a POL is thus a laboratory that provides the physician in-office laboratory testing services, thereby allowing the physician to have quicker access to laboratory results. Depending on the rules and regulations in any given state, the lab can be owned and operated either by a single physician practice or by more than one physician practice. In many states, additional regulations prohibit the acceptance of specimens from outside the clinician's practice.
Types of POLs and their workflow
POLs come in many different types. Nearly any practice can operate a POL. However, the most common types include gastroenterology, family practice, internal medicine, obstetrics and gynecology, and hematology and oncology practices. This is likely due to the need for these specialties to get quick results for treatment plan decisions.
According to United Healthcare's Oxford's In-Office Laboratory Testing and Procedures List (2012), the specialists that use an in-office laboratory include:
- Primary care physicians and specialists: In some parts of the world, this type of doctor is referred to as a general practitioner. This doctor is the first point of contact for a patient and coordinates referrals to other physicians as necessary (dependent upon the insurance plan a patient has). Often these doctors are family medicine or internal medicine specialists by training. In some cases, these doctors are OB/GYNs, nephrologists, allergists, pediatricians, or emergency medicine specialists.
- Dermatologists / dermatopathologists: A dermatologist is a doctor who specializes in skin conditions. Dermatologists can also board certify as dermatopathologists, which are trained in both dermatology and pathology. A dematopathologist examines tissue samples taken as part of a biopsy, for example.
- Rheumatologists: Rheumatologists deal with issues related to joints, soft tissue, autoimmune diseases, vasculitis, and hereditary connective tissue disorders.
- Urologists: Urologists examine issues related to the male and female urinary tract, as well as the male reproductive tract.
- Pediatricians: Pediatricians are trained to treat medical conditions found in infants, children, and adolescents. Generally, they do not see patients over the age of 18.
- Pulmonologists: Pulmonologists specialize in conditions related to the respiratory tract.
- Hematologists / oncologists / pediatric hematologists: Hematologists specialize in blood-related conditions, oncologists in cancers, and pediatric hematologists in childhood blood disorders.
- Obstetricians / gynecologists: Obstetricians specialize in conditions related to pregnancy. Often they are also gynecologists, specializing in women’s reproductive health conditions.
- Reproductive endocrinologists / infertility: This is a sub-branch of the above specialty, addressing both male and female infertility issues.
Other specialists such as surgeons are not as likely to have a physician office lab. Surgeries often take place in a hospital, and therefore tests would be processed through the hospital lab.
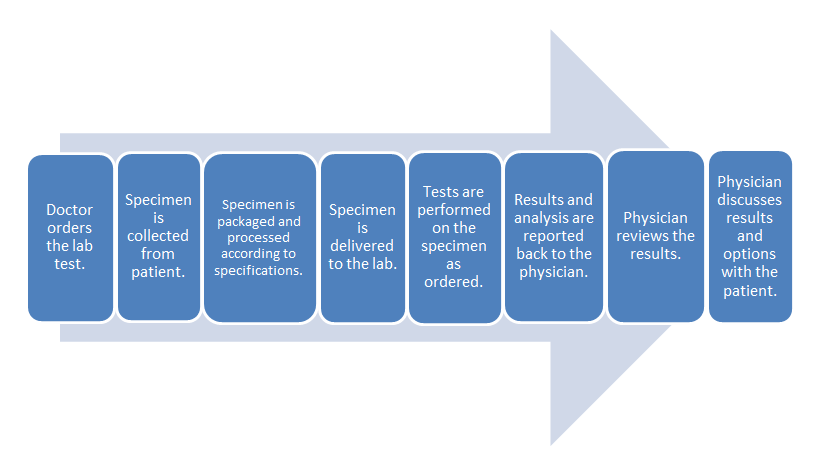
- Common clinical laboratory workflow:
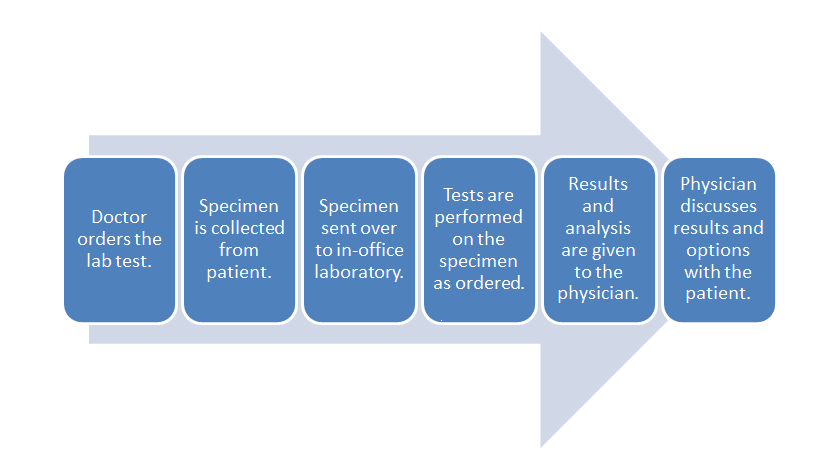
- POL workflow:
The difference in these two workflows mostly comes down to the time spent in transporting the specimen to an outside lab and waiting for the processing. The in-office lab saves time in those parts of the process.
History and market trends of the POL
In ancient times, patient diagnosis predicated on what the physician could observe during an exam of the patient, and in some cases the samples from the patient (Berger, 1999a).
Beginning in the Middle Ages and ending during the eighteenth century, bed side medicine was the predominant form of practice. Patients were diagnosed at their bed side, similar to what modern clinicians call point-of-care testing (POCT) (Berger, 1999a).
During the eighteenth and nineteenth centuries, with the rise of hospital medicine, laboratory medicine started to play a bigger role in the diagnosis of patients (Berger, 1999a).
Although, instruments such as the stethoscope and thermometer came into use at the end of the nineteenth century, the clinical laboratory did not become a standard part of medical practice until the twentieth century (Berger, 1999b).
Early diagnostic testing
Diagnosis of patients via techniques, such as the examination of bodily fluids to predict disease, can be traced back to Hippocrates in ancient Greece. Hippocrates instituted diagnostic criterion that included listening to the patient’s lungs, examining the urine of the patient, and observing the patient’s skin color (Berger, 1999a).
Blood in urine was linked to kidney failure in 50 AD, followed by another early clinician, Galen, identifying diabetes as “diarrhea of urine” and noting a normal relationship between fluid intake and urinary output in 180 AD (Berger, 1999a).
In 900 AD, Isaac Judaeus established a protocol for using urine in patient diagnoses (Berger, 1999a). By 1300 AD, examination of the urine under a microscope (uroscopy) had become so popular it was nearly universal in Europe (Berger, 1999a).
The seventeenth century saw many innovations in diagnostic techniques as a result of the advances in literature related to the structure of the body and the formation of scientific societies (Berger, 1999a). Some of the innovations that came from the seventeenth century include first attempts to use pulse and temperature as indications of illness in a patient; intravenous drug injections; and identification of the sweet taste of urine in patients with diabetes (Berger, 1999a).
In the eighteenth century, Dr. William Hewson began to identify ways to measure coagulants in blood tests, an event that set the stage for modern diagnostic laboratory practice (Berger, 1999a). During this time period, the ability to use temperature and blood pressure as diagnostic indicators was refined, allowing James Currie to treat his typhoid fever patients by putting them in a cold bath (Berger, 1999a).
Other advances out of the eighteenth century include Sir John Floyer’s pulse measuring technique, Tichy’s urine analysis technique, Dobson’s ability to prove the sweetness of both blood and urine in patients with diabetes was caused by sugar, and Home's development of a yeast test for sugar in urine (Berger, 1999a).
The nineteenth century is sometimes referred to as the era of public health; during this time independent laboratories started to develop (Berger, 1999a). In the United States, laboratory medicine was viewed with skepticism, as a destructive force related to medical knowledge (Berger, 1999a). As a result of this, many American physicians went to Europe to receive training on laboratory techniques (Berger, 1999a).
As older physicians retired from practice and faculty positions, opposition to laboratory practice faded, allowing for bacteriological discoveries like pasteurization (Berger, 1999a). The nineteenth century saw aseptic methodologies produce fewer deaths after surgery, resulting in a greater emphasis on hygienic practices (Berger, 1999a). This is also the period of time when x-ray and microscopy become more important to the practice of medicine (Berger, 1999a).
Around 1850, the laboratory became popularized, and the first hospital laboratories begin to appear (Berger, 1999a). Prior to this time period, most laboratory tests were performed at the bed side or were performed by the physician in the office laboratory (Berger, 1999a).
Diagnostic testing in the twentieth century
Although the nineteenth century was a time of advancement for clinical laboratory practice, the blossoming of the clinical laboratory did not occur until the twentieth century (Berger, 1999b). In the early twentieth century, laboratories began to stratify into the different types of sub-specialties seen in practice today: public health, forensic, and clinical (Berger, 1999b).
In 1928, when Alexander Fleming accidentally discovered penicillin, he ushered in the age of antibiotics, which allowed for new treatment options for infections, especially when combined with Domagk’s discovery that sulphonomides had antibacterial attributes and did not harm humans (Berger, 1999b).
In the twentieth century, laboratory medicine personnel needed to be certified and licensed as the movement to ensure quality in medicine came to the laboratory (Berger, 1999b). Organizations were founded to accommodate this, including the American Society for Clinical Pathology (ASCP), founded in 1922 to offer certifications to pathologists (Berger, 1999b).
By the end of the 1950s, the clinical laboratory had earned the respect of other medical professionals and the public, ensuring professional legitimacy (Berger, 1999b). This was accomplished by the discoveries made in the clinical laboratory, leading to new treatment options for patients, who would have endured difficult illnesses, or died, without such interventions (Berger, 1999b).
The initial creation of Medicare in 1965 was seen as an opportunity for free money by the healthcare industry as a whole. As costs increased, loopholes were found to get more reimbursements; however, these loopholes would be closed, and the resourceful provider would find other ways to continue the practice of charging more to get a higher reimbursement (Berger, 1999c).
The government soon discovered the potential for fraud and abuse inherent in Medicare. The need for regulations in order to prevent such abuses became apparent as early as 1967 (Berger, 1999c). The Clinical Laboratory Improvement Act came into effect that year as a tool to regulate laboratories practicing across state lines, and it was amended in 1988 to include nearly all laboratories practicing in the United States (Berger, 1999c).
In 1989, an estimated 98,400 POLs were operating in the United States. Estimates from the time vary from 20,000 to 200,000 due to the lack of a standard definition for a POL and the need for physicians to self-report the status of their lab (U.S. Department of Health and Human Services [HHS], 1989). Some of these issues continue to persist today, as states often have different definitions for a POL.
In 1989, limited regulatory controls existed for POLs, resulting in wide variations in the complexity of testing among POLs (HHS, 1989). Kusserow, writing for the HHS Office of Analysis and Inspections, noted the following during this time period:
Physicians operating office laboratories conduct approximately 25 percent of all laboratory testing in the country. Sixteen States have laws pertaining to them. About $20 billion is spent nationally on laboratory services annually, of which POLs receive $5 billion. Each year Medicare pays POLs over $400 million (HHS, 1989).
Kusserow also found the average 1985 Medicare Part B payment to POLs was $7 per test, compared to $10 per test for an independent lab, or $19 per test to a lab classified as "other" (HHS, 1989). One can see the trend: the POL became a cheaper option when compared to other types of labs.
Since 1995, with a better understanding and acceptance of regulations on the laboratory and the list of waived tests growing from 8 to 40 (Bachman, 2004), the number of POLs in the United States increased to a total of 120,399 or 49% of all the laboratories in the United States as of December 2013 (Centers for Medicare and Medicaid Services [CMS], 2013a). Additionally, 60% of the POLs in the United States today are running Clinical Laboratory Improvement Amendments (CLIA) waived tests, and 24% hold provider performed microscopy (PPM) certificates (CMS, 2013b).
According to Bachman (2004), POL growth is expected to increase in the future due to an aging population of baby boomers with money to finance laboratory testing and an increased interest and awareness in healthcare topics. Other factors to watch out for in the future are a softening stance on testing by payors (an example of this is the addition of an initial physical exam given to new Medicare beneficiaries for preventative care) and additions to the list of CLIA waived tests (Bachman, 2004).
Why do POLs exist?
Historically, POLs are a subset of point-of-care testing (POCT), laboratory testing that is done where the patient is located. These laboratories came about initially because as clinical medicine became more complicated to practice and techniques became more sophisticated, physicians still needed to perform tests to diagnose patients.
POLs also exist because the industry was looking for a cheaper way to test. Running a POL was found to be an effective way to provide clinician information for treatment plans, while at the same time saving insurers money.
In some cases, POLs opened to provide additional revenue streams for a physician’s practice. The reasons for the POL's existence are varied, but the central reason is to provide quality diagnoses, treatment, and care to patients in the healthcare system.
Advantages and disadvantages of running a POL
In the early days of the POL, lack of regulation proved to be a disadvantage; however, over time, have caught up for the better.
Advantages include
- quicker access to test results for the clinician, leading to more treatment options for the patient;
- greater efficiency of the clinical workflow;
- cheaper testing, though subject to individual test and pricing information; and
- patient comfort and happiness, including time saved by having to go only to one location.
Disadvantages include:
- the physician office being the only point-of-access, with some physicians not willing to release patient information to an outside party (such as a hospital or competing clinician). This disadvantage may be eliminated due to regulatory changes in April of 2014, now allowing patients direct access to their laboratory results;
- patients not feeling comfortable about the physician's office being the central repository of information, and physicians may not see the value in having a lab in their practice; and
- the cost of meeting compliance requirements for local, state, and federal regulations, especially in states with stricter requirements.
These lists are of course limited; one could weigh advantages and disadvantages endlessly if the appropriate time was spent to fully evaluate the endeavor. Some of this process would be related to the individual practice in question.
How the POL integrates with the entire practice
POLs can integrate with an entire practice in a variety of ways. First, POLs can store laboratory data in a form more readily exchanged between the laboratory and the patient's broader electronic health record (EHR). A slight disconnect often exists between reference labs and physician offices. By placing a lab in the physician office, tighter integration of patient and testing data is achieved, a benefit for both the patient and the practitioner.
The tighter integration may save patients follow-up visits for diagnoses that are able to be done in the office laboratory. For example, in diagnosing a urinary tract infection, the physician office can significantly reduce time spent sending the sample off for testing by doing the testing independently.
Additionally, the POL can allow the financial departments of a practice to track costs and revenue by using laboratory data. For example, during flu season the physician can budget more money for gloves if their data indicates they're seeing more patients during this time.
Laboratory data can also assist with trends related to a population. If a POL notices an unexpected trend in disease among the patient population, the lab data can help the entire organization decide how best to address the related issues through community education or some other outreach program.
POL or reference lab?
On average, most POL testing is simple and basic, falling under what is known as Clinical Laboratory Improvement Amendments (CLIA) waived tests. These tests will be discussed further in the next section, but for now know they are near the patient and simple to perform. As previously discussed, bringing simple laboratory testing to the physician office provides benefits to both the patient and the physician, making the POL more attractive.
For some physicians a POL is best because of their location; they may operate in a rural environment and would not have access to laboratory services if they did not do it themselves. For others, the expense of creating such a lab has swayed them towards using a reference lab, which can perform complex tests and, in many cases, has a staff available 24/7. And while placing a POL in the physician office may integrate patient and lab data better, software-based offerings like Health Gorilla that provide real-time results may be sufficient for physicians that prefer to use a reference lab.
In the end, the decision to set up a POL or use a reference lab is based on a review of the advantages and disadvantages, finding a balance of what is best for both the patients' interest and the practice's long-term stability.