Difference between revisions of "Template:Article of the week"
Shawndouglas (talk | contribs) (Updated article of the week text) |
Shawndouglas (talk | contribs) (Updated article of the week text.) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Heinen BMCBioinfo2020 21.png|240px]]</div> | ||
'''"[[Journal: | '''"[[Journal:HEnRY: A DZIF LIMS tool for the collection and documentation of biospecimens in multicentre studies|HEnRY: A DZIF LIMS tool for the collection and documentation of biospecimens in multicentre studies]]"''' | ||
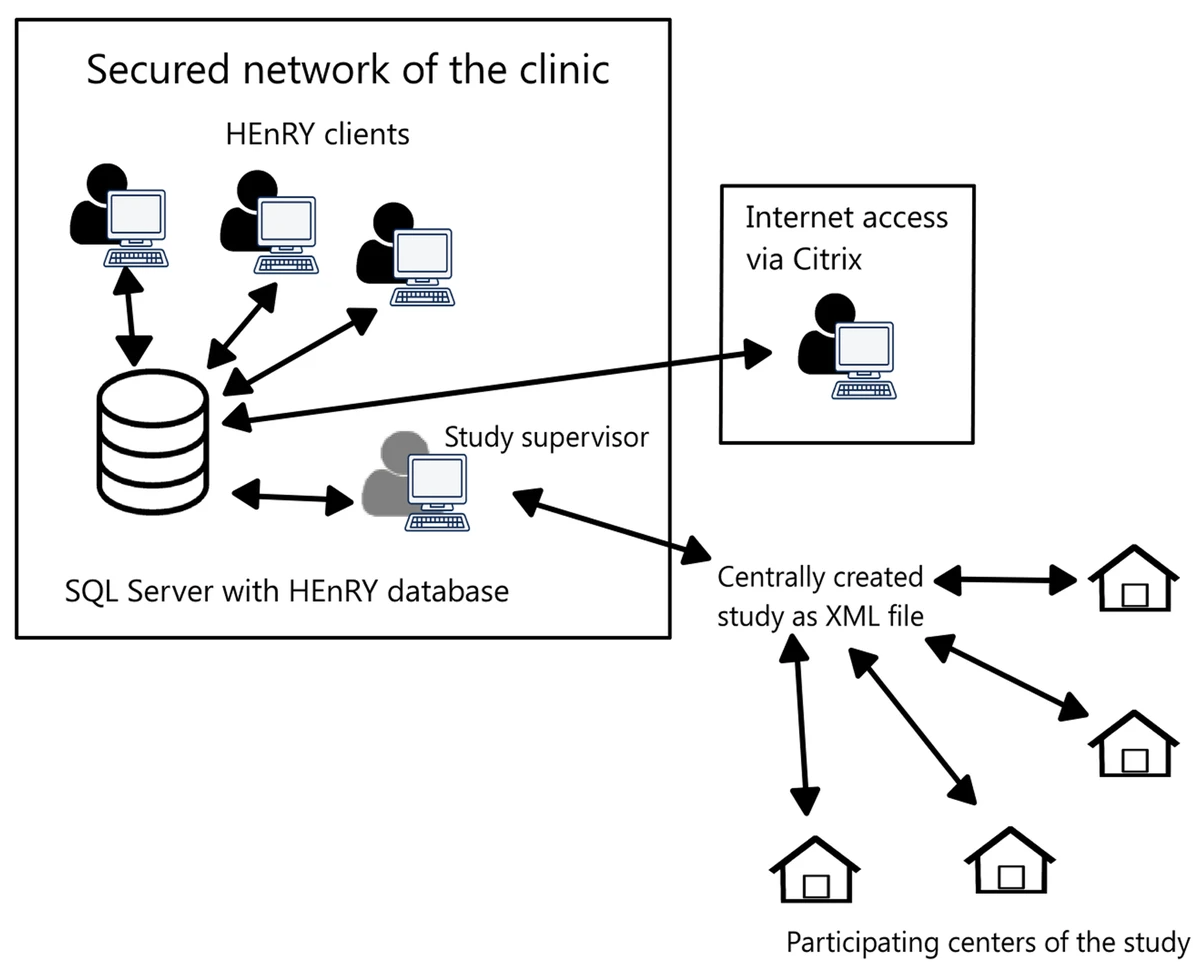
Well-characterized biological specimens (biospecimens) of high quality have great potential for the acceleration of and quality improvement in [[Translational research|translational biomedical research]]. To improve accessibility of local [[Sample (material)|specimen]] collections, efforts have been made to create central repositories ([[biobank]]s) and catalogues. Available technical solutions for creating professional local specimen catalogues and connecting them to central systems are cost intensive and/or technically complex to implement. Therefore, the HIV-focused Thematic Translational Unit (TTU) of the German Center for Infection Research (DZIF) developed a [[laboratory information management system]] (LIMS) called HIV Engaged Research Technology (HEnRY) for implementation into the HIV Translational Platform (TP-HIV) at the DZIF and other research networks. ('''[[Journal:HEnRY: A DZIF LIMS tool for the collection and documentation of biospecimens in multicentre studies|Full article...]]''')<br /> | |||
<br /> | <br /> | ||
''Recently featured'': | ''Recently featured'': | ||
: ▪ [[Journal:Bringing big data to bear in environmental public health: Challenges and recommendations|Bringing big data to bear in environmental public health: Challenges and recommendations]] | |||
: ▪ [[Journal:Enzyme immunoassay for measuring aflatoxin B1 in legal cannabis|Enzyme immunoassay for measuring aflatoxin B1 in legal cannabis]] | |||
: ▪ [[Journal:The regulatory landscape of precision oncology laboratory medicine in the United States: Perspective on the past five years and considerations for future regulation|The regulatory landscape of precision oncology laboratory medicine in the United States: Perspective on the past five years and considerations for future regulation]] | : ▪ [[Journal:The regulatory landscape of precision oncology laboratory medicine in the United States: Perspective on the past five years and considerations for future regulation|The regulatory landscape of precision oncology laboratory medicine in the United States: Perspective on the past five years and considerations for future regulation]] | ||
Revision as of 19:01, 3 August 2021
"HEnRY: A DZIF LIMS tool for the collection and documentation of biospecimens in multicentre studies"
Well-characterized biological specimens (biospecimens) of high quality have great potential for the acceleration of and quality improvement in translational biomedical research. To improve accessibility of local specimen collections, efforts have been made to create central repositories (biobanks) and catalogues. Available technical solutions for creating professional local specimen catalogues and connecting them to central systems are cost intensive and/or technically complex to implement. Therefore, the HIV-focused Thematic Translational Unit (TTU) of the German Center for Infection Research (DZIF) developed a laboratory information management system (LIMS) called HIV Engaged Research Technology (HEnRY) for implementation into the HIV Translational Platform (TP-HIV) at the DZIF and other research networks. (Full article...)
Recently featured:
- ▪ Bringing big data to bear in environmental public health: Challenges and recommendations
- ▪ Enzyme immunoassay for measuring aflatoxin B1 in legal cannabis
- ▪ The regulatory landscape of precision oncology laboratory medicine in the United States: Perspective on the past five years and considerations for future regulation