Template:Article of the week
From LIMSWiki
Revision as of 16:02, 14 July 2021 by Shawndouglas (talk | contribs) (Updated article of the week text)
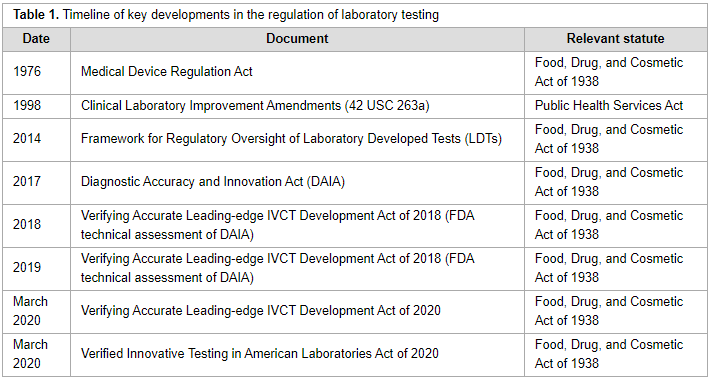
The regulatory landscape for precision oncology in the United States is complicated, with multiple governmental regulatory agencies with different scopes of jurisdiction. Several regulatory proposals have been introduced since the Food and Drug Administration released draft guidance to regulate laboratory developed tests in 2014. Key aspects of the most recent proposals and discussion of central arguments related to the regulation of precision oncology laboratory tests provides insight to stakeholders for future discussions related to regulation of laboratory tests. (Full article...)
Recently featured:
- ▪ Institutional ELN-LIMS deployment: Highly customizable ELN-LIMS platform as a cornerstone of digital transformation for life sciences research institutes
- ▪ Health care and cybersecurity: Bibliometric analysis of the literature
- ▪ Epidemiological data challenges: Planning for a more robust future through data standards