Difference between revisions of "Journal:A high-throughput method for the comprehensive analysis of terpenes and terpenoids in medicinal cannabis biomass"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 634: | Line 634: | ||
|} | |} | ||
===Accuracy=== | |||
To determine method accuracy, three replicates of a sample preparation were spiked with 0.5, 0.25, and 0.05 mg/g of CT and sabinene and extracted as described above. Unspiked biomass was extracted to determine endogenous levels. The recovery requirements of the spike as expressed in % adjusted for endogenous levels were 80–120%. Measured values ranged from 61.8% to 121.4%, with the majority of compounds ranging from 80% to 120% recovery across the three concentrations (Table 3). | |||
{| | |||
| STYLE="vertical-align:top;"| | |||
{| class="wikitable" border="1" cellpadding="5" cellspacing="0" width="50%" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" colspan="4"|'''Table 3.''' Recovery of analytes in spiked sample at three concentration levels | |||
|- | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;"| | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;" colspan="3"|% Spike Recovery | |||
|- | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;"|Compound | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;"|High | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;"|Mid | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;"|Low | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|α-Pinene (+/−) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|106.4 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|108.2 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|97.5 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Camphene | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|112.6 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|121.4 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|119.0 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Sabinene | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|108.8 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|115.7 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|98.7 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|β-Pinene (+/−) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|110.7 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|113.7 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|102.2 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Myrcene | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|106.5 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|104.8 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|95.5 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|3-Carene | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|112.4 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|118.7 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|115.7 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|α-Terpinene | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|78.8 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|84.5 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|81.8 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|''p''-Cymene | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|112.2 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|115.6 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|116.5 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Limonene | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|110.6 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|114.6 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|107.9 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Eucalyptol | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|116.6 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|109.9 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|119.2 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Ocimene | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|101 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|105.7 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|98.6 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|γ-Terpinene | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|104.6 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|108.9 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|101.6 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Terpinolene | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|97.1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|100.4 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|93.0 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Linalool | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|113.6 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|114.5 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|105.5 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Isopulegol | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|61.8 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|66.2 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|82.6 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|trans-Caryophyllene | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|93.5 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|99.1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|89.7 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Humulene | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|92.5 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|98.3 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|90.7 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|β-''cis''-Caryophyllene | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|62.0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|72.9 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|87.7 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Caryophyllene Oxide | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|69.0 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|76.3 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|84.8 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Guaiol | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|102.6 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|104.1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|93.3 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|α-Bisabolol | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|98.7 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|100.3 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|93.5 | |||
|- | |||
|} | |||
|} | |||
==References== | ==References== | ||
Revision as of 16:12, 28 July 2020
| Full article title | A high-throughput method for the comprehensive analysis of terpenes and terpenoids in medicinal cannabis biomass |
|---|---|
| Journal | Metabolites |
| Author(s) | Krill, Christian; Rochfort, Simone; Spangenberg, German |
| Author affiliation(s) | AgriBio, the Centre For AgriBioscience |
| Primary contact | Email: christian dot krill at agriculture dot vic dot gov dot au |
| Year published | 2020 |
| Volume and issue | 10(7) |
| Article # | 276 |
| DOI | 10.3390/metabo10070276 |
| ISSN | 2218-1989 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.mdpi.com/2218-1989/10/7/276/htm |
| Download | https://www.mdpi.com/2218-1989/10/7/276/pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
|
|
This article contains rendered mathematical formulae. You may require the TeX All the Things plugin for Chrome or the Native MathML add-on and fonts for Firefox if they don't render properly for you. |
Abstract
Cannabis and its secondary metabolite content have recently seen a surge in research interest. Cannabis terpenes and terpenoids in particular are increasingly the focus of research efforts due to the possibility of their contribution to the overall therapeutic effect of medicinal cannabis. Current methodology to quantify terpenes in cannabis biomass mostly relies on large quantities of biomass, long extraction protocols, and long gas chromatography (GC) gradient times, often exceeding 60 minutes. They are therefore not easily applicable in the high-throughput environment of a cannabis breeding program. The method presented here, however, is based on a simple hexane extract from 40 mg of biomass, with 50 μg/mL dodecane as internal standard, and a gradient of less than 30 minutes. The method can detect 48 individual terpenes and terpenoids and was validated for selectivity, linearity, limit of detection/limit of quantitation (LOD/LOQ), precision, intermediate precision, and accuracy (recovery) for 22 terpenes and terpenoids. The validation parameters are comparable to previously published studies that employ significantly longer runtimes and/or more complex extraction protocols. It is currently being applied to medicinal cannabis precision breeding programs.
Keywords: cannabis volatiles, terpenes, terpenoids, profiling, high-throughput method
Introduction
Cannabis is a genus of annual dioecious plants within the family Cannabaceae with a rich and complex constituency of pharmacologically relevant phytochemicals.[1][2] Under the Single Convention on Narcotic Drugs (1961), cannabis was deemed to be a plant without medicinal purpose, a conclusion based on very little scientific evidence or clinical trial data.[3] As a result, medicinal use of cannabis was practically prohibited by its status as an illegal narcotic worldwide, and research into cannabis chemistry and biology was largely limited to law enforcement and forensics applications.[4] Changes in the attitudes towards cannabis across various societies and increasing evidence for the benefits of cannabinoids in the treatment of otherwise intractable conditions have precipitated recent changes to legislature in a number of jurisdictions, including Australia.[3] Interest in the medicinal uses of cannabis is increasing, as indicated by a growing number of review articles on the topic.[5][6][7][8]
Cannabis, cannabis extracts, and purified (to varying degrees) major cannabinoids such as Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are now being used for the treatment of intractable and debilitating illnesses, such as Dravet’s Syndrome, and investigated for a range of others.[5][7][9][10][11][12] When administered as medicinal cannabis extracts, that is, along with the remaining portion of other cannabinoids and non-cannabinoid phytochemicals, less than a quarter of the equivalent dose of purified CBD is required, and markedly lower side effects are commonly observed.[7] This is in-line with a long line of anecdotal evidence that complex plant-based medicines are more effective than their isolated “actives,” which has given rise to the concept of the “entourage effect,” the modulating or synergistic effects of phytochemical compounds such as the minor cannabinoids on those effects related to THC.[13][14][15] Despite a current lack of understanding for the underlying mechanisms[16], the concept is increasingly applied to non-cannabinoid cannabis phytochemicals, such as terpenes and terpenoids.[6][9][10][17]
Terpenes are a class of hydrocarbons synthesized from the five-carbon (C5) “building blocks” dimethylallyl pyrophosphate and isopentenyl pyrophosphate. Depending on the number of C5 units and possible substitutions, they are further classified based on number of units (e.g., C10 monoterpenes [two sub-units], C15 sesquiterpenes [three sub-units]) or functional groups (terpenoids and oxygenated). Mono- and sesquiterpenes are classified as volatile and semi-volatile compounds, respectively, and higher order terpenes (e.g., C20 diterpenes and C30 triterpenes) exist as steroids, waxes, and resins. Cannabis mono- and sesquiterpenes are responsible for the characteristic smell of the plant and its products. They are also present at pharmacologically relevant concentrations[15][17][18][19], and a growing body of evidence suggests they have pharmacological properties in their own rights.[20][21] Their common use as food additives and in cosmetics products highlights the inherent safety of these compounds.[20]
As Leghissa and colleagues note[22], conclusively identifying terpenes in cannabis is challenging due to the large variety of possible candidates and a lack of commercial standards for a large number of them. With the increased interest in medicinal cannabis and the contribution of terpenes to the entourage effect, methods to quantify terpenes in medicinal cannabis biomass and products are becoming more available in the literature.[23][24][25][26] Often, these methods require large quantities of biomass (1–5 g), use large quantities of organic solvents (up to 100 mL/sample), and include separations with gradient runtimes exceeding 60 minutes. These methods are therefore not easily applied in a high-throughput manner. To facilitate terpene quantitation and profiling in order to enable accelerated precision breeding programs in medicinal cannabis, we developed a fast (30-minute separation), microscale (40 mg sample), high-throughput gas chromatography–mass spectrometry (GC-MS) terpene profiling and quantitation method.
Results
Sampling techniques
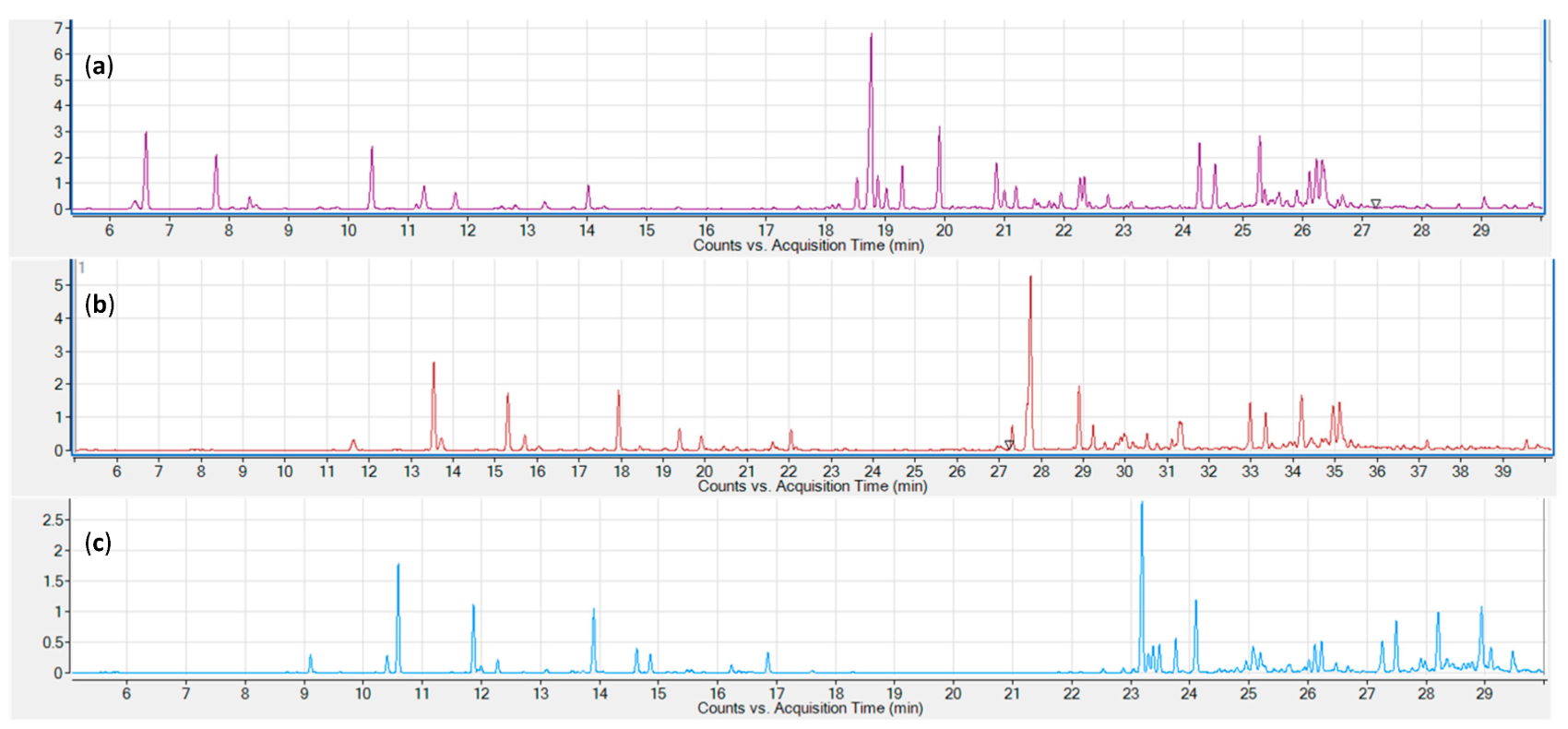
Three common sampling and sample introduction techniques were evaluated: static headspace, solid-phase microextraction (SPME), and liquid injection of an organic solvent extract. Representative chromatograms for the three tested sampling techniques are shown in Figure 1. All three techniques provide excellent signal strengths for the lower boiling monoterpenes. Sesquiterpenes are underrepresented in the static headspace chromatogram (a), while the SPME chromatogram shows a stronger signal for early eluting sesquiterpenes. Higher boiling sesquiterpenes are only adequately represented in the hexane extract chromatogram.
|
Columns
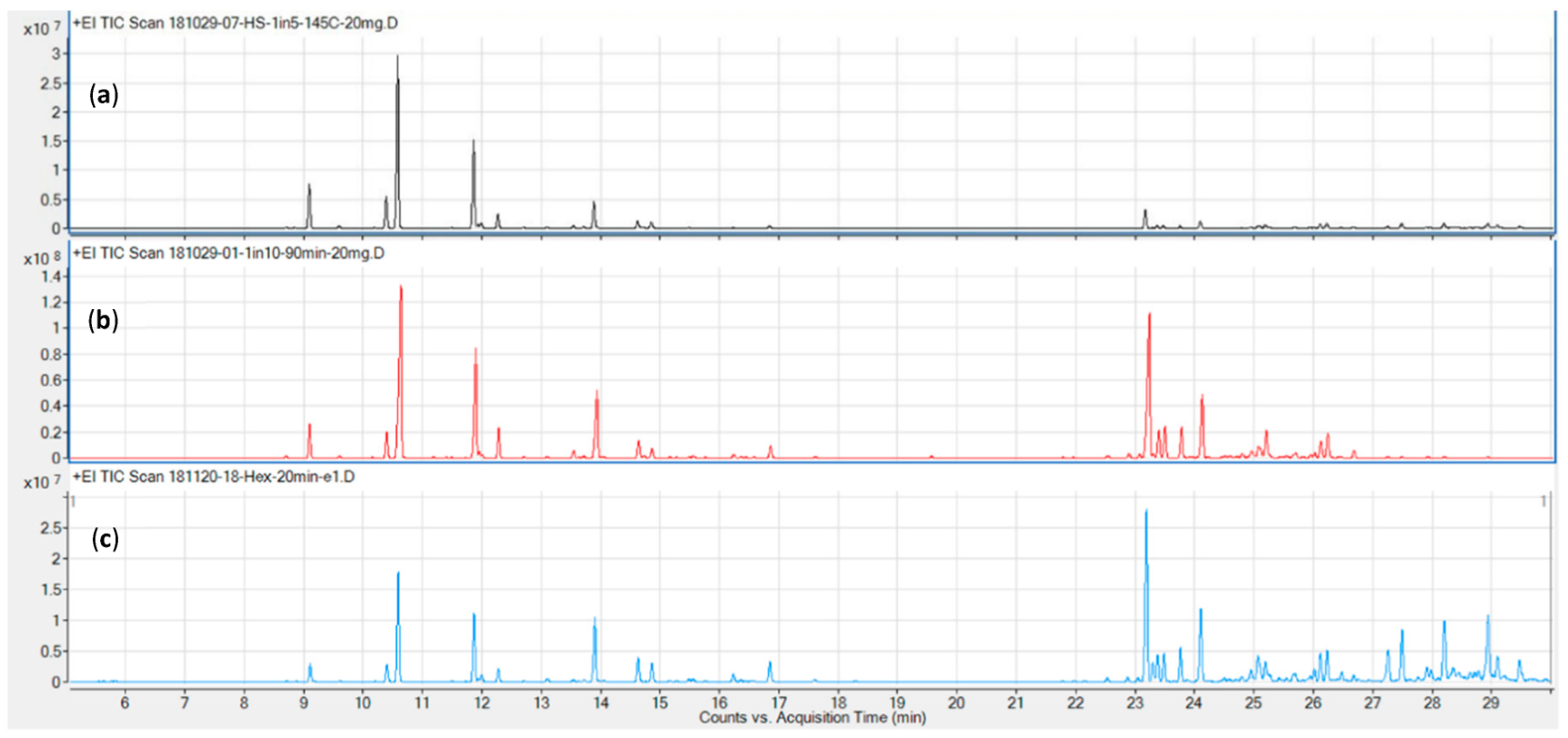
Representative chromatograms for the three columns tested (DB5, DB17, and VF35, in order of increasing polarity) are shown in Figure 2. The DB5 (c) and DB17 (a) columns performed comparably well in terms of resolution and overall run time (≤ 30 minutes), while the VF 35 column required a runtime in excess of 35 minutes for the chosen temperature program.
|
Solvent optimisation
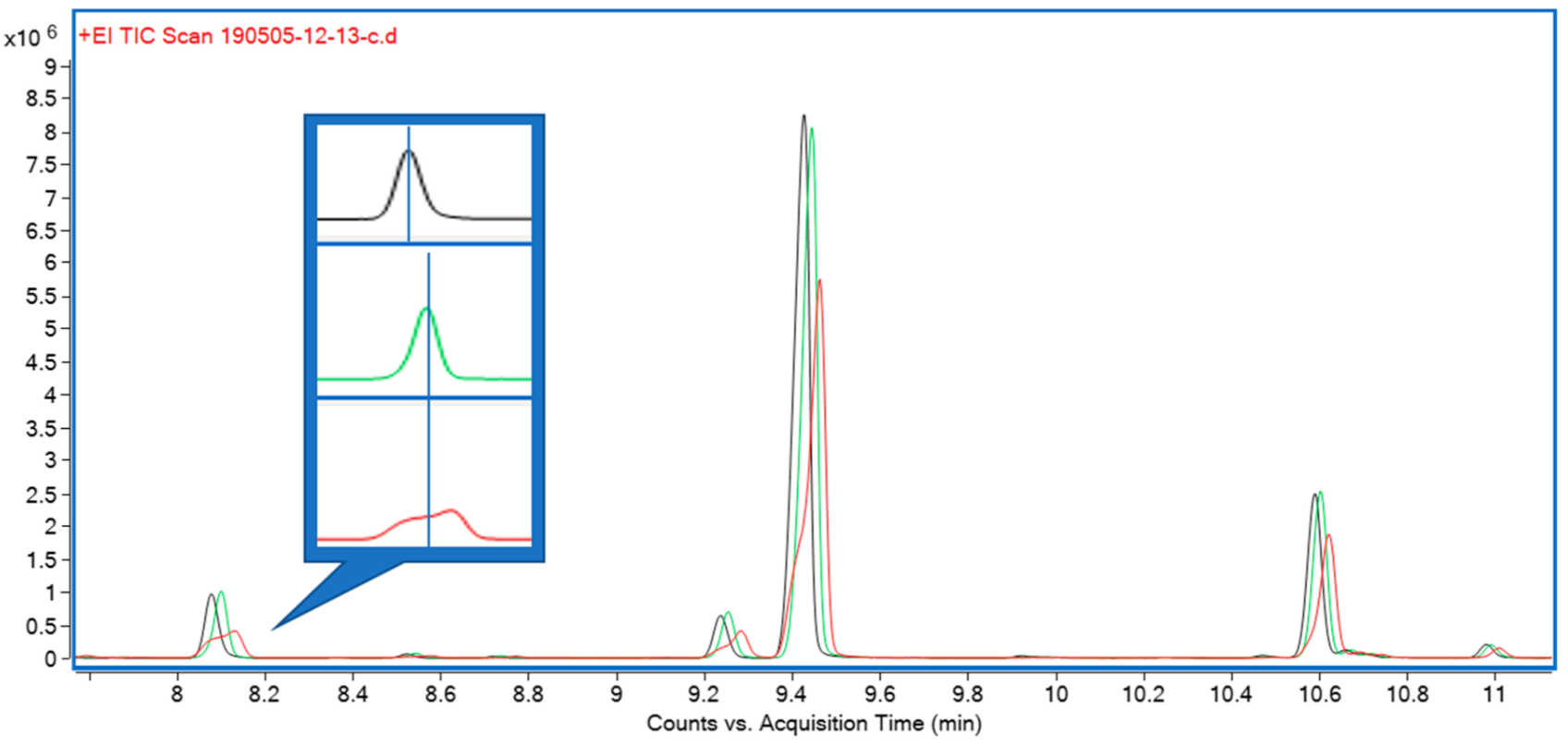
Three solvents—hexane, isopropanol, and ethyl acetate—were initially trialed for liquid extraction. All three performed equally well in terms of extraction efficiency, but both isopropanol and, to a lesser extent, ethyl acetate were causing early eluting compounds to show undesirable peak shapes during gas chromatography (GC) analysis. A comparison of peak shapes is shown in Figure 3. Furthermore, hexane extracts appeared as light green, clear extracts, whereas ethyl acetate and isopropanol extracts were opaque and of a much darker color, indicating the presence of a higher proportion of non-target compounds.
|
Compound identification and resolution
A total of 48 individual compounds were detected across multiple strains used. Of those, 22 were monoterpenes and -terpenoids, with the remainder consisting of sesquiterpenes and terpenoids. Compound names, base peak (quantifier) ion m/z, retention times and indices, and identification status are summarized in Table 1. Most monoterpene identities predicted by spectral library matching were confirmed using authentic standards, with the exception of fenchol and trans-2-pinanol. The α thujene, β-phellandrene, and thujanol identities were confirmed by matching published retention times indices (RIs). Sesquiterpene preliminary identities were confirmed using authentic standards for eight out of 25 compounds (Table 1), and RI matching for a further three.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Resolution was calculated based on the following equation (discussed further in the "Materials and methods" section):
Values presented in Table 1 are based on the respective base peak (qualifier) ion and are given for the closest preceding (Rp) and following peak (Rs).
Linearity
A combined cannabis terpenes (CT) and sabinene standard was injected at ten concentrations from 100 μg/mL to 0.195 ng/mL, based on a 1:2 dilution series of the 100 μg/mL standard. All calibration curves obtained were linear with R2 values greater than or equal to 0.994. Based on individual detection limits, the curves spanned all ten calibration points, except linalool, ocimene, and trans-caryophyllene (100–0.391 μg/mL); caryophyllene oxide, guaiol, and isopulegol (100–1.56 μg/mL); and α-bisabolol and β-cis-caryophyllene (100–12.5 μg/mL). Nerolidol was removed from the analysis due to poor peak shapes and high detection limits.
Detection and quantitation limits
Limit of detection (LOD) and limite of quantitation (LOQ) were determined as LOD = 3.3 × σ/S and LOQ = 10 × σ/S, with σ representing the standard deviation of the compound and S the slope of the compound’s calibration curve. Detection limits range from 0.017 μg/mL to 1.144 μg/mL (Table 2); quantitation limits range from 0.052 to 3.468 μg/mL (β-pinene and α-bisabolol for both LOD/LOQ).
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Accuracy
To determine method accuracy, three replicates of a sample preparation were spiked with 0.5, 0.25, and 0.05 mg/g of CT and sabinene and extracted as described above. Unspiked biomass was extracted to determine endogenous levels. The recovery requirements of the spike as expressed in % adjusted for endogenous levels were 80–120%. Measured values ranged from 61.8% to 121.4%, with the majority of compounds ranging from 80% to 120% recovery across the three concentrations (Table 3).
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- ↑ Russo, E.B. (2019). "The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No "Strain," No Gain". Frontiers in Plant Science 9: 1969. doi:10.3389/fpls.2018.01969. PMC PMC6334252. PMID 30687364. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6334252.
- ↑ Russo, E.B. (2007). "History of cannabis and its preparations in saga, science, and sobriquet". Chemistry & Biodiversity 4 (8): 1614-48. doi:10.1002/cbdv.200790144. PMID 17712811.
- ↑ 3.0 3.1 Wright, R. (24 November 2017). "Cannabis Industry Report" (PDF). EverBlu Capital. https://www.everblucapital.com/wp-content/uploads/2017/11/EverBlu-Research-Cannabis-Industry-Report.pdf. Retrieved 12 January 2019.
- ↑ Whiting, P.F.; Wolff, R.F.; Deshpande, S. et al. (2015). "Cannabinoids for Medical Use: A Systematic Review and Meta-analysis". JAMA 313 (24): 2456–73. doi:10.1001/jama.2015.6358. PMID 26103030.
- ↑ 5.0 5.1 Klumpers, L.E.; Thacker, D.L. (2019). "A Brief Background on Cannabis: From Plant to Medical Indications". Journal of AOAC International 102 (2): 412–20. doi:10.5740/jaoacint.18-0208. PMID 30139415.
- ↑ 6.0 6.1 Booth, J.K.; Bohlmann, J. (2019). "Terpenes in Cannabis sativa - From plant genome to humans". Plant Science 284: 67–72. doi:10.1016/j.plantsci.2019.03.022. PMID 31084880.
- ↑ 7.0 7.1 7.2 Pamplona, F.A.; da Silva, L.R.; Coan, A.C. (2018). "Potential Clinical Benefits of CBD-Rich Cannabis Extracts Over Purified CBD in Treatment-Resistant Epilepsy: Observational Data Meta-analysis". Frontiers in Neurology 9: 759. doi:10.3389/fneur.2018.00759. PMC PMC6143706. PMID 30258398. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6143706.
- ↑ Bonini, S.A.; Premoli, M.; Tambaro, S. et al. (2018). "Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history". Journal of Ethnopharmacology 227: 300–15. doi:10.1016/j.jep.2018.09.004. PMID 30205181.
- ↑ 9.0 9.1 Weston-Green, K. (2019). "The United Chemicals of Cannabis: Beneficial Effects of Cannabis Phytochemicals on the Brain and Cognition". In Costain, W.J.; Laprarie, R.B.. Recent Advances in Cannabinoid Research. IntechOpen. doi:10.5772/intechopen.79266. ISBN 9781838806415.
- ↑ 10.0 10.1 Baron, E.P. (2018). "Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science". Headache 58 (7): 1139–86. doi:10.1111/head.13345. PMID 30152161.
- ↑ Rohleder, C.; Müller, J.K.; Lange, B. et al. (2016). "Cannabidiol as a Potential New Type of an Antipsychotic. A Critical Review of the Evidence". Frontiers in Pharmacology 7: 422. doi:10.3389/fphar.2016.00422. PMC PMC5099166. PMID 27877130. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5099166.
- ↑ Bonn-Miller, M.O.; ElSohly, M.A.; Loflin, M.J.E. et al. (2018). "Cannabis and cannabinoid drug development: evaluating botanical versus single molecule approaches". International Review of Psychiatry 30 (3): 277–84. doi:10.1080/09540261.2018.1474730. PMC PMC6242809. PMID 30179534. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6242809.
- ↑ Ben-Shabat, S.; Fride, E.; Sheskin, T. et al. (1998). "An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity". European Journal of Pharmacology 353 (1): 23–31. doi:10.1016/s0014-2999(98)00392-6. PMID 9721036.
- ↑ Mechoulam, R.; Ben-Shabat, S. (1999). "From gan-zi-gun-nu to anandamide and 2-arachidonoylglycerol: The ongoing story of cannabis". Natural Product Reports 16 (2): 131–43. doi:10.1039/a703973e. PMID 10331283.
- ↑ 15.0 15.1 McPartland, J.M.; Russo, E.B. (2001). "Cannabis and Cannabis Extracts: Greater Than the Sum of Their Parts?". Journal of Cannabis Therapeutics 1 (3–4): 103–32. doi:10.1300/J175v01n03_08.
- ↑ Santiago, M.; Sachdev, S.; Arnold, J.C. etc. (2019). "Absence of Entourage: Terpenoids Commonly Found in Cannabis sativa Do Not Modulate the Functional Activity of Δ 9-THC at Human CB 1 and CB 2 Receptors". Cannabis and Cannabinoid Research 4 (3): 165-176. doi:10.1089/can.2019.0016. PMC PMC6757242. PMID 31559333. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6757242.
- ↑ 17.0 17.1 Russo, E.B. (2011). "Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects". British Journal of Pharmacology 163 (7): 1344–64. doi:10.1111/j.1476-5381.2011.01238.x. PMC PMC3165946. PMID 21749363. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3165946.
- ↑ Kamal, B.S.; Kamal, F.; Lantela, D.E. (2018). "Cannabis and the Anxiety of Fragmentation-A Systems Approach for Finding an Anxiolytic Cannabis Chemotype". Frontiers in Neuroscience 12: 730. doi:10.3389/fnins.2018.00730. PMC PMC6204402. PMID 30405331. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6204402.
- ↑ Adams, T.B.; Taylor, S.V. (2010). "Safety evaluation of essential oils: A constituent-based approach". In Baser, K.H.C.; Buchbauer, G.. Handbook of Essential Oils: Science, Technology, and Applications. CRC Press. pp. 185–208. ISBN 9781420063158.
- ↑ 20.0 20.1 Nuutinen, T. (2018). "Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus". European Journal of Medicinal Chemistry 157: 198–228. doi:10.1016/j.ejmech.2018.07.076. PMID 30096653.
- ↑ do Vale, T.G.; Furtado, E.C.; Santos Jr., J.G. et al. (2002). "Central effects of citral, myrcene and limonene, constituents of essential oil chemotypes from Lippia alba (Mill.) n.e. Brown". Phytomedicine 9 (8): 709–14. doi:10.1078/094471102321621304. PMID 12587690.
- ↑ Leghissa, A.; Hildenbrand, Z.L.; Schug, K.A. (2018). "A review of methods for the chemical characterization of cannabis natural products". Journal of Separation Science 41 (1): 398-415. doi:10.1002/jssc.201701003. PMID 28986974.
- ↑ Giese, M.W.; Lewis, M.A.; Giese, L. et al. (2015). "Development and Validation of a Reliable and Robust Method for the Analysis of Cannabinoids and Terpenes in Cannabis". Journal of AOAC International 98 (6): 1503–22. doi:10.5740/jaoacint.15-116. PMID 26651562.
- ↑ Ibrahim, E.A.; Wang, M.; Radwan, M.M. et al. (2019). "Analysis of Terpenes in Cannabis sativa L. Using GC/MS: Method Development, Validation, and Application". Planta Medica 85 (5): 431–38. doi:10.1055/a-0828-8387. PMID 30646402.
- ↑ Fischedick, J.T.; Hazekamp, A.; Ekelens, T. et al. (2010). "Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes". Phytochemistry 71 (17–18): 2058–73. doi:10.1016/j.phytochem.2010.10.001. PMID 21040939.
- ↑ Fischedick, J.T. (2017). "Identification of Terpenoid Chemotypes Among High (-)- trans-Δ 9- Tetrahydrocannabinol-Producing Cannabis sativa L. Cultivars". Cannabis and Cannabinoid Research 2 (1): 34–47. doi:10.1089/can.2016.0040. PMC PMC5436332. PMID 28861503. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5436332.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. The original includes an "in prep" citation at citation 8, but the citation appears to be about grapes and tobacco and not related to cannabis; it could not be verified as published and has been omitted for this version.