Journal:Cannabinoid, terpene, and heavy metal analysis of 29 over-the-counter commercial veterinary hemp supplements
| Full article title | Cannabinoid, terpene, and heavy metal analysis of 29 over-the-counter commercial veterinary hemp supplements |
|---|---|
| Journal | Veterinary Medicine: Research and Reports |
| Author(s) | Wakshlag, Joseph J.; Cital, Stephen; Eaton, Scott J.; Prussin, Reece; Hudalla, Christopher |
| Author affiliation(s) | Cornell University College of Veterinary Medicine, ElleVet Sciences, ProVerde Laboratories |
| Primary contact | Email: Dr dot joesh at gmail dot com |
| Editors | Lyoo, Young |
| Year published | 2020 |
| Volume and issue | 11 |
| Page(s) | 45–55 |
| DOI | 10.2147/VMRR.S248712 |
| ISSN | 2230-2034 |
| Distribution license | Creative Commons Attribution-NonCommercial 3.0 Unported |
| Website | https://www.dovepress.com/cannabinoid-terpene-and-heavy-metal-analysis-of-29-over-the-counter-co |
| Download | https://www.dovepress.com/getfile.php?fileID=57398 (PDF) |
Abstract
Purpose: The use of veterinary low-tetrahydrocannabinol (THC) Cannabis sativa (i.e., hemp) products has increased in popularity for a variety of pet ailments. Low-THC Cannabis sativa is federally legal for sale and distribution in the United States, and the rise in internet commerce has provided access to interested consumers, with minimal quality control.
Materials and methods: We performed an internet word search of “hemp extract and dog” or “CBD product and dog” and analyzed 29 products that were using low-THC Cannabis sativa extracts in their production of supplements. All products were tested for major cannabinoids, including ∆9-tetrahydrocannabinol (THC), cannabidiol (CBD), cannabigerol (CBG), and other minor cannabinoids, as well as their respective carboxylic acid derivatives tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), and cannabigerolic acid (CBGA) using an ISO/IEC 17025-certified laboratory. Products were also tested for major terpenes and heavy metals to understand constituents in the hemp plants being extracted and distributed.
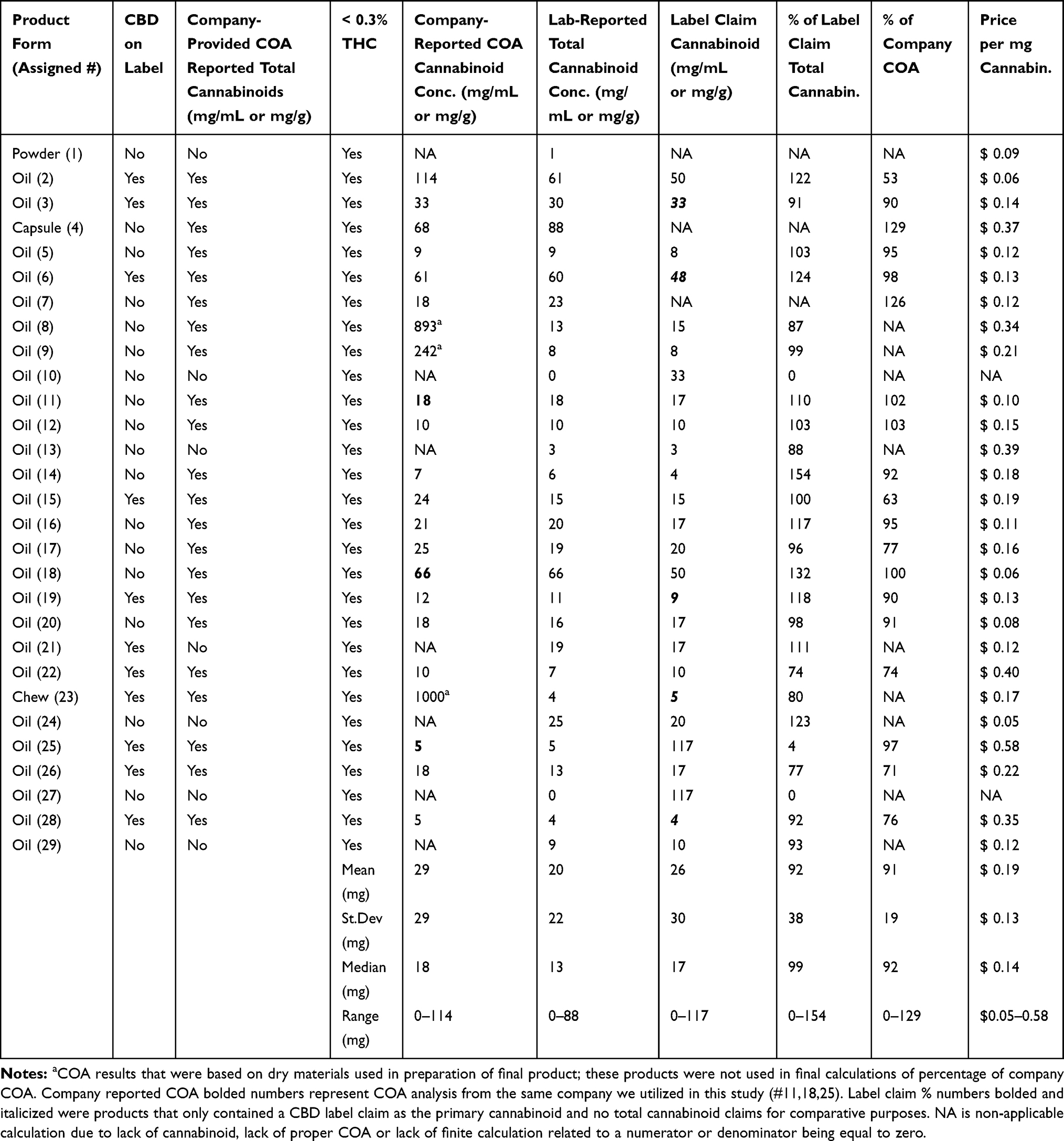
Results: All products were below the federal limit of 0.3% THC, with variable amounts of CBD (0– 88 mg/mL or g). Only two products did not supply a CBD or total cannabinoid concentration on their packaging or website, while 22 of 29 had an associated certificate of analysis (COA) from a third-party laboratory. Ten of the 27 products were within 10% of the total cannabinoid concentrations of their label claim, with a median concentration of 93% of claims (0– 154%). Heavy metal contamination was found in four of 29 products, with lead being the most prevalent contaminant (three of 29).
Conclusion: The products analyzed had highly variable concentrations of CBD or total cannabinoids, with only 18 of 29 being appropriately labeled according to current Food and Drug Administration (FDA) non-medication, non-dietary supplement, or non-food guidelines. Owners and veterinarians wanting to utilize CBD-rich Cannabis sativa products should be aware of low-concentration products and should obtain a COA enabling them to fully discuss the implications of use and calculated dosing before administering to pets.
Keywords: cannabinoid, hemp, supplement, cannabidiol, pet, terpene, oral
Introduction
The recent federal legalization and deregulation of low-tetrahydrocannabinol (THC) Cannabis sativa, otherwise known as hemp, as a commercial crop in the United States has created a new supplement market for humans and pets alike that is largely unregulated.[1] The de-scheduling of low-THC Cannabis sativa-derived extracts forced any oversight of products containing hemp-derived cannabidiol (CBD), and other cannabinoids, to the Food and Drug Administration (FDA).[2] The lack of clear FDA regulations and inconsistent state regulations being implemented leaves many practitioners contemplating the legality of low-THC Cannabis sativa distribution in each state, even though federally legal. Some associations and organizations refer to the Dietary Supplement Health and Education Act of 1994 (DSHEA) for guidelines regarding marketing and labeling of Cannabis sativa-derived CBD products, when in fact the U.S. Congress clarified the intent of DSHEA as not relevant to animals.[3] Instead, this lack of oversight responsibility has left a legal gray zone where animal supplements are not illegal, but are self-regulated with enforcement discretion maintained by the FDA. The FDA currently only oversees three defined categories when it comes to animal products: medicines, medical devices, and food.
Currently, at the time of writing, compliant labeling and marketing of low-THC CBD products must not state or imply the prevention, mitigation, or curing of disease. This mandate mirrors all other human or animal supplements and nutraceuticals on the market today. Until the FDA resolves the issue regarding guidelines of “hemp” CBD products, many manufacturers will likely continue illegal and dishonest marketing and labeling, possibly weighing the earning potential against the unlikely event of FDA enforcement in a saturated market.
The use of CBD-rich extracts on pets is commonplace, as identified by Kogan et al. in a range of survey work, leaving veterinarians in a tenuous place as health professionals, particularly due to the paucity of clinical or safety studies. Client survey work suggests that CBD-rich Cannabis extracts are currently being used to treat a variety of disorders, including anxiety, cancer and cancer chemotherapy side effects, inflammatory bowel disease, osteoarthritis, and seizures.[4][5] CBD is the primary cannabinoid of interest due to the tremendous amount of pre-clinical and human clinical research suggesting it may have utility in a range of inflammatory and neurologic disease processes.[6][7][8][9][10] Other cannabinoids can also be found in many of these preparations, including ∆9-tetrahydrocannabinol (THC), ∆8-tetrahydrocannabinol (∆8-THC), cannabichromene (CBC), cannabinol (CBN), cannabigerol (CBG), cannabidivarin (CBDV), exo-THC, tetrahydrocannabivarin (THCV), and all their derived acids, such as tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), and cannabigerolic acid (CBGA), as well as terpenes. Terpenes are a class of mono- and dicyclical volatile compounds that lead to the aroma of the extract and may also have modest medicinal properties, but they are typically found at lower concentrations than cannabinoids (less than 1% dry weight of plant material).[11]
Unfortunately, due to a lack of regulation, quality control is suspect in all human and animal supplements, Cannabis and non-Cannabis supplements alike. Two recent publications examining selected cannabinoid concentrations in human over-the-counter products showed a tremendous disparity between labeling claims and analysis of the products, with over 40% having less than the labeled amount and over 40% having more than the labeled amount.[12][13] The THC concentrations in a Canadian study were less than 0.01% for all products showing compliance with the Canadian standard for Cannabis-derived CBD products.[12] In a study examining 14 European products, all with THC concentrations being below the 0.2% allowable limit, CBD concentrations varied from the labeled amount of total cannabinoids or CBD concentrations.[14]
The cause for this sort of disparity in products can be from a range of issues, including batch to batch variation, intentional improper labeling, degradation over time, poor extraction techniques, and lack of certification of the laboratories being used to measure cannabinoids. That said, in the veterinary literature, there has been some initial pilot pharmacokinetic, safety, and clinical trials that provide some insights for veterinarians regarding dosing regimens.[15][16][17][18][19]
The primary objective of this study were to provide information regarding the important plant constituents—including cannabinoids, terpenes, and heavy metals contamination (arsenic, cadmium, lead and mercury)—in commercial products obtained through internet commerce, using liquid chromatography with diode array detection and mass spectrometry (LC-DAD/MS), headspace gas chromatography with flame ionization detection (HS-GC-FID), and inductively coupled plasma mass spectrometry (ICP-MS), respectively. The cannabinoids analyzed included CBD, THC, ∆8-THC, CBG, CBN, CBC, THCV, CBDV, and the derived acids CBDA, THCA, and CBGA. Major terpenes in the analysis included β-myrcene, linalool, limonene, β-caryophyllene, pinenes, and other lesser terpenes. Secondary objectives were to examine labels to determine if manufacturers complied with current FDA supplement guidelines, examine the relative consistency between analyzed concentrations versus labeled CBD or total cannabinoids, and examine the manufacturer’s ability to produce a certificate of analysis regarding cannabinoid analysis for the lot purchased.
Materials and methods
Product selection and preparation
Pet-specific products were obtained from an internet search, which included the Google search engine input of “hemp extract and dog” or “CBD product and dog.” The first 30 products out of 65 products specifically for dog use were identified and often came in multiple forms (dry capsule, oil tincture, soft chew, or powder). Products were not purchased if the advertisements were from hemp seed rather than whole plant extract. If multiple forms were identified, then an oil product was chosen for analysis. If an oil was not available, then a powdered capsule form was chosen, and if a chew was the only form available, then it was chosen for analysis. All products were paid for in U.S. dollars, and the retail price was recorded minus the shipping and handling costs.
After purchase all companies were contacted to provide a certificate of analysis (COA) related to the product purchased based on lot number. If an original third-party COA was not provided, then marketing material or label concentrations were used to assess against laboratory analysis by a certified third-party laboratory. After mixing well, three separate 2 mL or 2 g aliquots were prepared for analysis within a month of receipt (ensuring they were within the labeled expiratory date) and were then sent to an ISO/IEC 17025-certified laboratory (ProVerde, Milford, MA, USA) for analysis of products for common cannabinoids, including CBD, CBDA, CBDV, THC, THCA, ∆8-THC, exo-THC, THCV, CBC, CBG, CBGA, and CBN. Products were also tested for a range of common terpenes found in Cannabis, including camphene, β-pinene, 3-carene, α-terpinene, α-pinene, ocimene, limonene, P-cymene, eucalyptol, γ-terpinene, terpinolene, linalool, β-myrcene, β-caryophyllene, humulene, caryophyllene oxide, and α-bisabolol. Lastly, one aliquot from each product was tested for four major heavy metals found in cannabis, including arsenic, cadmium, lead, and mercury.
Cannabinoid analysis
All samples submitted for testing were visually and microscopically inspected prior to analysis for foreign material, with no remarkable findings. Samples were homogenized in their entirety. Solid samples were mechanically reduced to a free-flowing powder, while oil samples were vortexed for one minute prior to subsampling. Aliquots for testing were made either at 20, 100, or 1000 mg to achieve a lower detection limit of 0.0025 wt% for chewables and 0.01 wt% for orals. Cannabinoids were extracted into either isopropanol, acetonitrile, or a 60/40 (vol/vol) mixture of acetonitrile and water, filtered to 0.2 µm and diluted in a 60/40 (vol/vol) mixture of acetonitrile and water prior to quantitation.
Chromatography was achieved using a Waters ACQUITY H-Class ultra-performance liquid chromatography (UPLC) system with diode array detector (DAD) and quadrupole mass spectrometer. The system was calibrated for 12 cannabinoids, including seven major cannabinoids (CBGA, CBG, CBDA, CBD, THCA, THC, and CBN) and five minor cannabinoids (exo-THC, ∆8-THC, CBC, THCV and CBDV) using five-point linear regression over the range of 0.0005–0.05 mg/mL, with a minimum coefficient of determination of 0.999 using 1/X weighting. Quantitation utilized the 225 nm extracted absorbance from the 3-D DAD spectra (190–500 nm), with confirmed peaks compared to reference library UV spectra as well as mass fragmentation patterns from 200–400 m/z for identification.
Terpene analysis
All terpene samples followed the homogenization and subsampling procedures described in the cannabinoid analysis section. Aliquots of nominally 20 mg, irrespective of matrix type, were placed in 20 mL borosilicate headspace vials. Samples were analyzed for terpene profiles using headspace gas chromatography with flame ionization detection (HS-GC-FID). An Agilent 7694 headspace autosampler was used for sample injection and utilizing nitrogen vial pressurization and carrier gas. A heated transfer line carried analytes to a split injection port of a Shimadzu GC-2014 gas chromatograph. Split ratio was maintained at a constant 10:1 ratio under column velocity control, with overall nitrogen flow rate of approximately 80 mL/min. The instrument was calibrated to analyze 16 terpene compounds using a six-point linear calibration over the range of 0.625– 37.5 µg (31–1875 ppm), with a minimum coefficient of determination of 0.98.
Heave metals analysis
Heavy metals were determined utilizing an Agilent 7800 inductively coupled plasma mass spectrometer (ICP-MS). Samples were homogenized with approximately 100 mg of sample and aliquoted into 25 mL MARSXpress microwave digestion tubes. After the addition of 2 mL of a 9:1 concentrated mixture nitric and hydrochloric acid in water, the samples were digested with microwave assist (CEM, Mars6) at 210°C for 20 minutes and allowed to cool prior to centrifuging and filtering. The resulting digest was diluted to a final volume of 20 mL with 0.5% hydrochloric acid in water prior to analysis. The analyzer was calibrated using a six-point linear calibration from 0 ppb to 5.00 ppb using 71 element standard mix. Continuing calibration verifications were performed every five to ten samples.
Results
Of the original 30 products identified as hemp extract-containing products that were purchased, all but one used hemp extract in the formulation of the product. One product was labeled as a “hemp chew,” but the ingredients contained no hemp-derived cannabinoid but rather only hemp seed oil. This product was excluded from the analysis since it is well known that hemp seed contains nominal cannabinoids and terpenes.[20] Of the 29 products that were analyzed, two were powders and one was a soft chew format; the remaining 26 were oil tinctures.
Labeling and COA
All of the boxes and labels were examined according to FDA compliant supplement guidelines, to determine any reference to cannabidiol or CBD concentration in the product, as well as for claims of mitigating a specific ailment. Eleven of the 29 products had reference to CBD as a constituent of their product, while the remaining products referred to their concentrations as “total cannabinioids” or “total hemp extract” (Table 1). A COA was available from 22 of the 29 manufacturers; however, three of the COAs were likely from the raw materials rather than the actual final oil tincture (represented by a in Table 1); therefore, those were considered to have a flawed COA, taking the number of actual product COAs to 19 of the 29 manufacturers. When examining the analysis of total cannabinoids of the product (which were predominantly CBD), the total cannabinoid concentration calculation was divided by the cost of the bottle (minus shipping), revealing a mean cost per mg of cannabinoid at $0.19/mg (median $0.14/mg – range $0.05-$0.58/mg); this calculation did not account for the two products that had no cannabinoid present on analysis (Table 1).
|
Cannabinoid analysis compared to the COA provided by the manufacturer was calculated as a mean percent and median of the COA (mean 75%, median 90%, range 0–129%), showing that COA concentrations were often lower than the actual certified laboratory analysis. These calculations were performed without the three companies with inappropriate COAs being factored into this calculation (19/29 products). Cannabinoid analysis compared to the label claim appeared to be more accurate (26/29 with label claims; mean 93%; median 99% – range 0–154%). Three of the manufacturer’s provided COAs were generated by the laboratory chosen for our comprehensive analysis, and our cannabinoid results were identical (bolded results on Table 1 – column labeled "Company-Reported COA Cannabinoid Conc.").
Cannabinoid concentrations
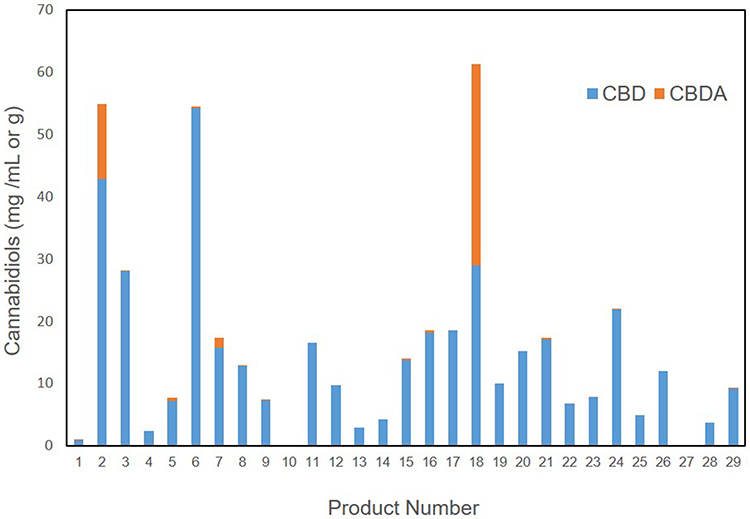
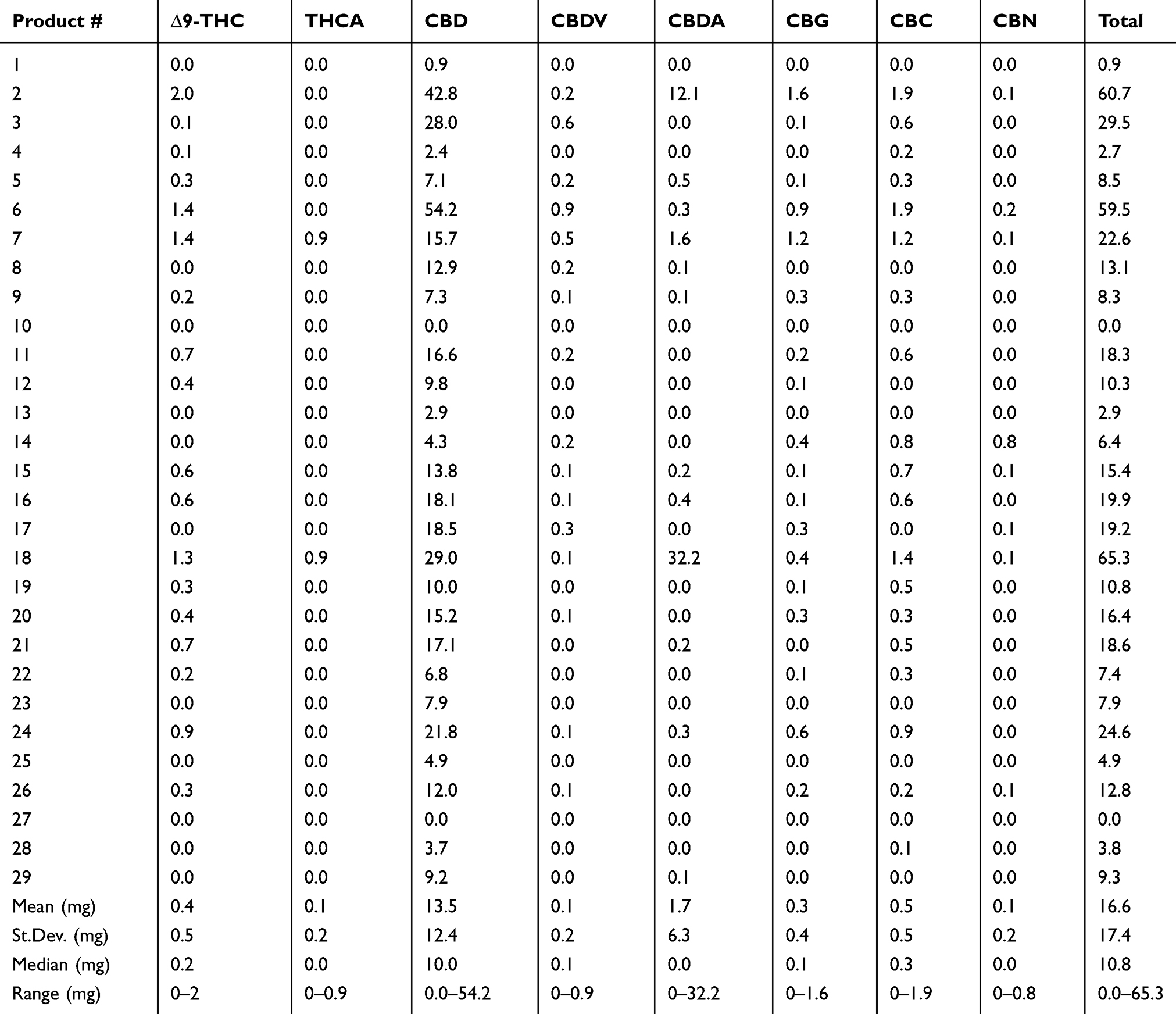
The cannabinoids that were identified in products based on mean abundance (and presence in products) in descending order were CBD (27 of 29 products), CBDA (12 of 29 products), CBC (19 of 29 products), CBG (18 of 29 products), ∆9-THC (19 of 29 products), THCA (two of 29 products), CBDV (17 of 29 products), and CBN (eight of 29 products). There was no detection of ∆8-THC, exo-THC, or CBGA in any of the products tested. As the major cannabinoids marketed in low-THC Cannabis sativa, CBD (and CBDA) concentrations are shown in Figure 1, revealing a large degree of variability in CBD and CBDA concentrations. Table 2 shows the entire cannabinoid profiles and reveals that all products are below the USDA limit of 0.3% dry weight (combined ∆9-THC and THCA).
|
|
Terpene concentrations
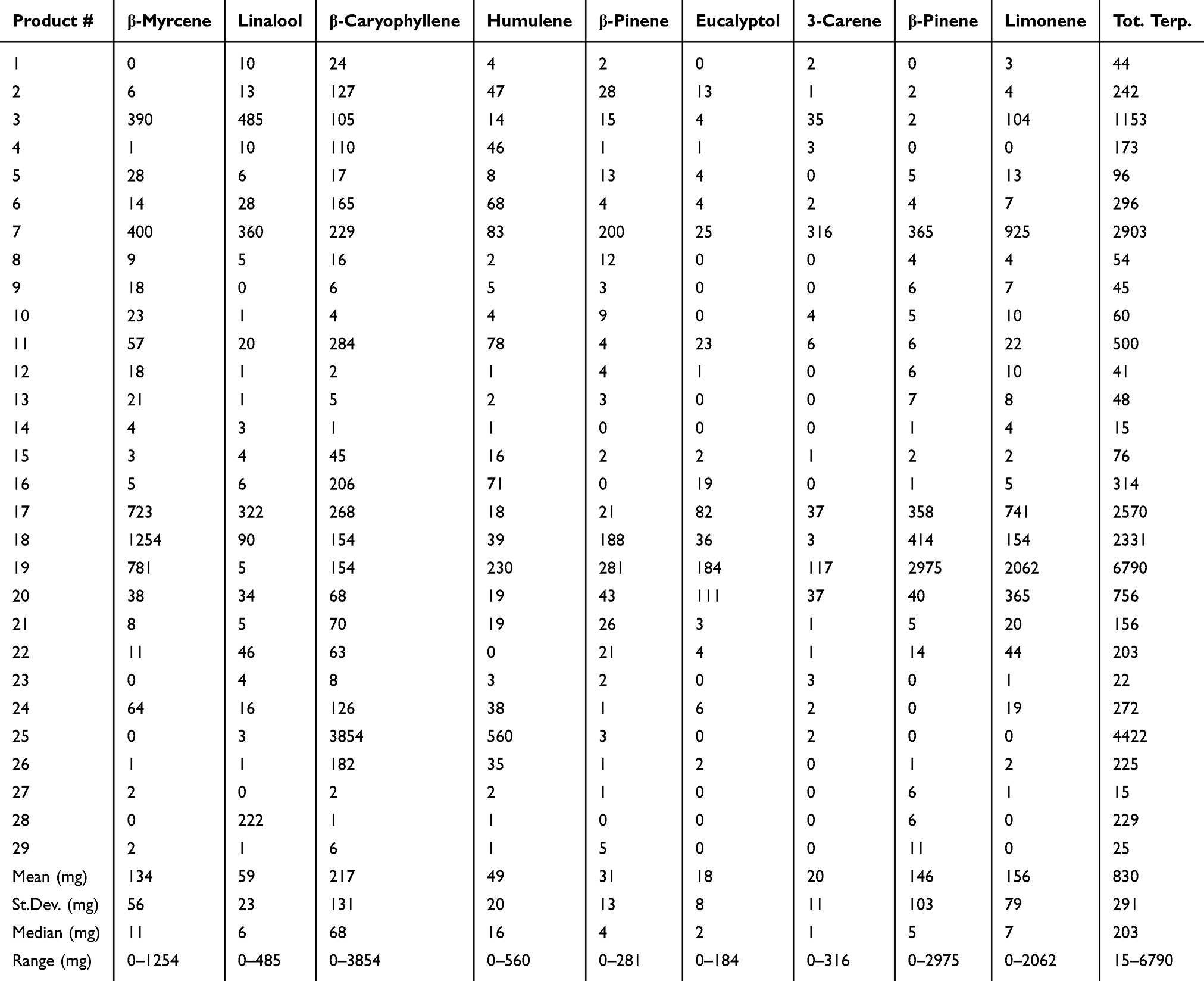
Analysis of terpenes revealed their concentration to be far lower than cannabinoids; the values are reported as mg/L or mg/kg. Although all of the analyzed terpenes could be found in some of the products at over 1 mg/kg, the only terpenes included in the table were those that could be found at over 100 mg/L or mg/kg in a product, representing the major terpenes found in hemp products (Table 3). Terpene abundance (and presence in products) based on mean concentrations, in descending order, were β-caryophyllene (29 of 29 products), humulene (28 of 29 products), β-myrcene (25 of 29 products), limonene (25 of 29 products), linalool (27 of 29 products), α-pinene (22 of 27 products), β-pinene (26 of 29 products), eucalyptol (19 of 29 products), and 3-carene (18 of 29).
|
Heavy metals concentrations
Of the 29 products analyzed, four were positive for heavy metals at above the lower limit of detection (products 1, 2, 23, and 24). Product one contained 2,104 µg/kg of arsenic, 209 µg/kg of cadmium, and 157 µg/kg of lead. Only arsenic was considered above the limit for oral consumption based on the laboratory analysis guidelines, and this product was a hemp powder supplement. Product 2 was an oil that contained 2,296 µg/mL of lead, which would not pass for oral consumption according to laboratory analysis guidelines and current United States Pharmacopeia (USP) guidelines. Products 23 and 24 were both oils that contained 262 µg/mL arsenic and 8 µg/mL of lead, respectively; both would be considered safe for oral consumption. No other products contained lead, mercury, arsenic, and cadmium at above the lower limit of detection for these heavy metals.
Discussion
This pet product examination is the first of its kind to utilize a certified laboratory in the analysis of cannabinoids, terpenes, and heavy metals in commercially available low-THC Cannabis sativa pet supplements. Prior work for human products has examined a smaller profile of cannabinoids in over-the-counter hemp products, showing numerous discrepancies with what was tested versus concentrations that were labeled.[12][13][14]
Our analysis showed that all products complied with THC requirements, containing less than 0.3% THC as either THC or THCA (the precursor acid to THC). These THC results were encouraging, ensuring relative safety from THC intoxication to pets using these products. Issues related to U.S. Department of Agriculture (USDA) regulation suggest that all products should be processed from certified “hemp farms,” yet this is difficult to determine as most companies could not supply us with paperwork related to their low-THC Cannabis sativa sourcing. Just over 75% of companies could supply us with a COA based on lot number of the product that was purchased, and three of those COAs were from the base low-THC Cannabis sativa utilized to prepare the product and not the final product itself. Obtaining a COA is an important part of understanding the necessary dosing of the product if using cannabinoids for wellness issues. It is also critical that veterinary staff become accustomed with interpreting COAs for pet owners. The presence of a heavy metals, residual solvents, or other contaminants does not automatically disqualify a product from being safe to use, but the product should at minimum comply with USP standards for orally consumed products. The fiscal nature of product selection shows that price paid per mg of cannabinoid can be a 10-fold difference depending on product choice. Interestingly, two of the 29 products had absolutely no cannabinoids detectable in the product, showing the lack of uniformity and fraud being perpetuated by some manufacturers.
Beyond the CBD and THC values represented, it is important to note that due to the extensive nature of our testing, we can confirm that there were no other forms of THC represented in any of the products (∆8-THC or exo-THC), which can still impart psychotropic properties.[21] Other cannabinoids of interest that may have implications in neurology and inflammation are CBC, CBG, and CBN, which could not be found in over 50% of products. In products that did contain some of these cannabinoids, the concentrations were less than 1 mg/mL or gram on average, with concentrations at high as 1.6 mg/mL in an occasional product. Further examination of the other THC and CBD forms, including THCA and CBDV, show that there is less than 1 mg/mL or gram on average and that these are not major cannabinoids found in products. More interestingly, CBDA was a major cannabinoid identified in two products. This is likely due to the use of lower temperatures during extraction and processing, which is not commonly found, as higher heat processing is typical, resulting in the decarboxylation conversions of the native CBDA to the neutral CBD.[20][22] It is likely that this is intentional, as CBDA has been associated with an anti-inflammatory effect and is thought to be involved in improved bioavailability of cannabinoids.[23][24][24][25] The remainder of the products and the two higher CBDA products also contained CBD as the primary cannabinoids, with a range of 0–54 mg/mL or gram and a median of 10 mg/mL or gram. In fact, there are few products that have over 20 mg/mL or gram (6 of 29) or 50 mg/mL (3 of 29) or gram of CBD or total cannabinoids. The implications of low concentration products is important since dosing appears to be in the 1–3 mg/kg range based on current knowledge[16][17][18], which would suggest that minimally 1 mL or gram of product would be needed per 10 kg body weight using products that are 20 mg/mL, while the products that are 50 mg/mL would only require 0.3 mL per 10 kg body weight. These concepts regarding dosing are necessary for veterinary health professionals to consider when discussing the logistics of safety, efficacy, value, and dosing surrounding product selection.
The fact that only 10 of the 29 products were within 90–110% of the label claims regarding cannabinoid concentrations provides little comfort to the consumer that products are labeled appropriately. Nine of the products were over 110% of the label claim on analysis, and 10 were under 90% of the label claim, with two of these containing no cannabinoids. This is not atypical of what has been found in human hemp products, where less than 50% were found to be within 90–110% of label claims.[13] Reasons for low-THC Cannabis sativa discrepancies involve many factors such as poor formulation, degradation of cannabinoids, and bioconversion over time, as well as inappropriate laboratory analysis. In addition, we did not test multiple batches of product, which may be another reason for drift in cannabinoid concentrations within certain products, bringing to light the need for further examination of such issues in the industry.
Currently, there are no established federal standards for low-THC Cannabis sativa testing. However, the ISO/IEC 17025 standard for analytical testing laboratories has become a requirement for labs providing testing services in multiple states, and it has become a foundational component of the recent interim USDA Hemp Farming and Testing guidelines.[26][27] Laboratories accredited to this standard are routinely assessed by accrediting bodies to ensure that laboratories adhere to good laboratory practices, utilizing validated methods on calibrated instruments. Analyst and technician training must be well documented to support the individual activities performed by laboratory personnel. Laboratories are also required to demonstrate their competency by participation in proficiency testing programs, in which they are challenged with blind samples to maintain accreditation. Results from this testing are collected with statistical comparison to other laboratories and/or to the established values for the testing performed. Practitioners and consumers should rely on product COAs provided only by an accredited laboratory to ensure that the data provided is reliable.
A further novelty of our investigation is not only the range of cannabinoids tested, but also the extensive terpene analysis of products. Of the over 20 terpenes assessed, there were only nine that had over 100 mg/kg of one or more of the terpenes in any one product (0.01%), reported in Table 3. The primary terpenes of interest were β-caryophyllene, β-myrcene, pinenes, humulene, linalool, and limonene; depending on the product, the profiles can be dramatically different. Terpenes are the major volatile products of hemp that provide a distinct odor. Interestingly, due to similar backbone precursor molecules, total terpenes can often, but do not always, follow cannabinoid concentrations. The enzymatic machinery of the plant cultivars do dictate terpene formation to a large degree (Table 3). Our data suggest that in some cases there were abnormally high total terpene concentrations in some products, which are likely due to manufacturers “spiking” products with terpenes to either provide some natural medicinal properties that have been attributed to terpenes[28][29], or to enhance the aroma, thereby misleading the consumer into thinking that the product was highly enriched with cannabinoids, despite phytocannabinoids having no scent or flavor.
Terpenes may have some therapeutic advantages; it has been observed that whole plant extracts can be superior to single molecule constituents, which is known as the “entourage effect.”[30][31][32][33] In general, the milligram quantity of total terpenes across products ranges from 0.015–6.7 mg/mL or gram, with only six products with over 1 mg/mL or gram of total terpenes. Further examination for any single terpene at concentrations higher than 1 mg/mL leaves only three products (product 18, 19, and 25) that have a single terpene at that level. Overall, total terpenes for most products would be similar in concentrations to some of the minor cannabinoids observed, making it difficult to elucidate exactly whether alternate cannabinoids and/or terpenes are exhibiting some of the synergy observed as the “entourage effect” discussed in the literature.
As with any plant material, accumulation of minerals from soil is part of the nutritional benefits of plant consumption; however, chronic consumption of any plant material with accumulation of heavy metals is an important health consideration. Most concerning is that hemp as a crop has been utilized in polluted areas to help with bioremediation of soils due to its ability to grow in heavily contaminated soils.[34][35][36] Crop growth in variable geographic regions leads to variable mineral and heavy metal accumulation. Lead and arsenic accumulation appear to be most relevant as potential contaminants leading to health concerns. Our analysis of four common heavy metals did show contamination in four of 29 products (12%). For two of these products, the recorded levels of arsenic and lead exceed the regulatory limits established by the Massachusetts Department of Public Health for cannabis products.[27] In one product with excessive arsenic accumulation, the product was a dried hemp powder (product 1). The product with excessive lead above the limit for oral consumption was an oil product (product 2). We cannot comment on whether this lead contamination was from the hemp, the carrier oil used as a diluent/solvent, or the processing equipment or materials used for extraction. Regardless, it becomes critical for pet owners to have products tested, or to insist on COA results for heavy metals before supplementation.
Our study did not examine other possible sources of contamination in pet hemp consumables, including solvents used in the extraction process, pesticides used on crops, mycotoxins that can accumulate in dried crops, and microbiological contaminants. For each of these issues there have been reports of contaminants in hemp production and extraction, which is why consumers must be aware and solicit this information from manufacturers.[36] The scope of this assessment was to study the constituents that might accumulate in the plant tissues themselves, not focusing on crop management or extraction related contamination, yet research assessing products for these contamination issues is sorely needed.
In summary, until further guidelines can be defined by the FDA, Federal Trade Commission, the USDA, and state governments, there is a need for intervention by veterinarians and technicians into this ever-expanding world of low-THC Cannabis sativa supplements. Practitioners need to become versed in product selection and utilization. They should also, minimally, be asking questions of manufacturers regarding CBD and THC concentrations in the products minimally, with further inquiries into potential contaminants including heavy metals, solvents, pesticides, microbials, and mycotoxins. The range and variability of products in the veterinary market is alarming, and veterinary professionals should only consider manufacturers providing product safety data such as COAs, pharmacokinetic data, and clinical application data when clients solicit information regarding product selection.
Acknowledgements
The authors would like to thank Ms. Amanda Howland for her help with the logistics involved in obtaining the products and results from ProVerde Labs.
Author contributions
All authors contributed to data analysis, drafting, or revising the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Conflicts of interest
Joseph J Wakshlag is a paid consultant of ElleVet Sciences and reports grants and personal fees from Ellevet Sciences, outside the submitted work. Stephen Cital and Reece Prussin are employed full-time at ElleVet Sciences, which researches, manufactures, and sells products containing cannabinoids. The authors report no other conflicts of interest in this work.
References
- ↑ "Public Law 115 - 334 - Agriculture Improvement Act of 2018". govinfo. United States Government Publishing Office. 20 December 2018. https://www.govinfo.gov/app/details/PLAW-115publ334.
- ↑ Food and Drug Administration (2020). "FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD)". Food and Drug Administration. https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd. Retrieved 27 March 2020.
- ↑ "S.784 - Dietary Supplement Health and Education Act of 1994". Congress.gov. 25 October 1994. https://www.congress.gov/bill/103rd-congress/senate-bill/784.
- ↑ Kogan, L.R.; Hellyer, P.W.; Robinson, N.G. (2016). "Consumers' Perceptions of Hemp Products for Animals" (PDF). Journal of the American Holistic Veterinary Medical Association 42 (Spring): 40–48. https://www.ahvma.org/wp-content/uploads/AHVMA-2016-V42-Hemp-Article.pdf.
- ↑ Kogan, L.R.; Hellyer, P.W.; Silcox, S. et al. (2019). "Canadian dog owners' use and perceptions of cannabis products". Canadian Veterinary Journal 60 (7): 749–55. PMC PMC6563876. PMID 31281193. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6563876.
- ↑ Izzo, A.A.; Borrelli, F.; Capasso, R. et al. (2009). "Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herbs". Trends in Pharmacological Sciences 30 (10): 515–27. doi:10.1016/j.tips.2009.07.006. PMID 19729208.
- ↑ White, C.M. (2019). "A Review of Human Studies Assessing Cannabidiol's (CBD) Therapeutic Actions and Potential". Journal of Clinical Pharmacology 59 (7): 923–34. doi:10.1002/jcph.1387. PMID 30730563.
- ↑ Landa, L.; Sulcova, A.; Gbelec, P. (2016). "The use of cannabinoids in animals and therapeutic implications for veterinary medicine: A review". Veterinarni Medicina 61: 111–22. doi:10.17221/8762-VETMED.
- ↑ Mastinu, A.; Ribaudo, G.; Ongaro, A. et al. (2020). "Critical Review on the Chemical Aspects of Cannabidiol (CBD) and Harmonization of Computational Bioactivity Data". Current Medicinal Chemistry. doi:10.2174/0929867327666200210144847. PMID 32039672.
- ↑ Premoli, M.; Aria, F.; Bonini, S.A. et al. (2019). "Cannabidiol: Recent advances and new insights for neuropsychiatric disorders treatment". Life Sciences 224: 120–7. doi:10.1016/j.lfs.2019.03.053. PMID 30910646.
- ↑ Pavlovic, R.; Nenna, G.; Calvi, L. et al. (2018). "Quality Traits of "Cannabidiol Oils": Cannabinoids Content, Terpene Fingerprint and Oxidation Stability of European Commercially Available Preparations". Molecules 23 (5): 1230. doi:10.3390/molecules23051230. PMC PMC6100014. PMID 29783790. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6100014.
- ↑ 12.0 12.1 12.2 Meng, Q.; Buchanan, B.; Zuccolo, J. et al. (2018). "A reliable and validated LC-MS/MS method for the simultaneous quantification of 4 cannabinoids in 40 consumer products". PLoS One 13 (5): e0196396. doi:10.1371/journal.pone.0196396. PMC PMC5931681. PMID 29718956. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5931681.
- ↑ 13.0 13.1 13.2 Bonn-Miller, M.O.; Liflin, M.J.E.; Thomas, B.F. et al. (2017). "Labeling Accuracy of Cannabidiol Extracts Sold Online". JAMA 318 (17): 1708–9. doi:10.1001/jama.2017.11909. PMC PMC5818782. PMID 29114823. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5818782.
- ↑ 14.0 14.1 Hazekamp, A. (2018). "The Trouble with CBD Oil". Medical Cannabis and Cannabinoids 1: 65–72. doi:10.1159/000489287.
- ↑ Łebkowska-Wieruszewska, B.; Stefanelli, F.; Chericoni, S. et al. (2019). "Pharmacokinetics of Bedrocan, a cannabis oil extract, in fasting and fed dogs: An explorative study". Research in Veterinary Science 123: 26–8. doi:10.1016/j.rvsc.2018.12.003. PMID 30580232.
- ↑ 16.0 16.1 Gamble, L.-J.; Boesch, J.M.; Frye, C.W. et al. (2018). "Pharmacokinetics, Safety, and Clinical Efficacy of Cannabidiol Treatment in Osteoarthritic Dogs". Frontiers in Veterinary Science 5: 165. doi:10.3389/fvets.2018.00165. PMC PMC6065210. PMID 30083539. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6065210.
- ↑ 17.0 17.1 Bartner, L.R.; McGrath, S.; Rao, S. et al. (2018). "Pharmacokinetics of cannabidiol administered by 3 delivery methods at 2 different dosages to healthy dogs". Canadian Journal of Veterinary Research 82 (3): 178–83. PMC PMC6038832. PMID 30026641. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6038832.
- ↑ 18.0 18.1 McGrath, S.; Bartner, L.R.; Rao, S. et al. (2019). "Randomized blinded controlled clinical trial to assess the effect of oral cannabidiol administration in addition to conventional antiepileptic treatment on seizure frequency in dogs with intractable idiopathic epilepsy". Journal of the American Veterinary Medical Association 254 (11): 1301–8. doi:10.2460/javma.254.11.1301. PMID 31067185.
- ↑ Deabold, K.A.; Schwark, W.S.; Wolf, L. et al. (2019). "Single-Dose Pharmacokinetics and Preliminary Safety Assessment with Use of CBD-Rich Hemp Nutraceutical in Healthy Dogs and Cats". Animals 9 (10): 832. doi:10.3390/ani9100832. PMC PMC6826847. PMID 31635105. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6826847.
- ↑ 20.0 20.1 Citti, C.; Pacchetti, B.; Vandelli, M. et al. (2018). "Analysis of cannabinoids in commercial hemp seed oil and decarboxylation kinetics studies of cannabidiolic acid (CBDA)". Journal of Pharmaceutical and Biomedical Analysis 149: 532–40. doi:10.1016/j.jpba.2017.11.044. PMID 29182999.
- ↑ Hine, B.; Toreelio, M.; Gershon, S. (1977). "Analgesic, heart rate, and temperature effects of delta8-THC during acute and chronic administration to conscious rats". Pharmacology 15 (1): 63–72. doi:10.1159/000136664. PMID 14347.
- ↑ Wang, M.; Wang, V.-H.; Avula, B. et al. (2016). "Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry". Cannabis and Cannabinoid Research 1 (1): 262–71. doi:10.1089/can.2016.0020. PMC PMC5549281. PMID 28861498. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5549281.
- ↑ Rock, E.M.; Limebeer, C.L.; Parker, L.A. (2018). "Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry". Effect of cannabidiolic acid and ∆ 9-tetrahydrocannabinol on carrageenan-induced hyperalgesia and edema in a rodent model of inflammatory pain 235 (11): 3259-3271. doi:10.1007/s00213-018-5034-1. PMID 30225659.
- ↑ 24.0 24.1 Takeda, S.; Misawa, K.; Yamamoto, I. et al. (2008). "Cannabidiolic acid as a selective cyclooxygenase-2 inhibitory component in cannabis". Drug Metabolism and Disposition 36 (9): 1917-21. doi:10.1124/dmd.108.020909. PMID 18556441.
- ↑ Eichler, M.; Spinedi, L.; Unfer-Grauwiler, S. et al. (2012). "Heat exposure of Cannabis sativa extracts affects the pharmacokinetic and metabolic profile in healthy male subjects". Planta Medica 78 (7): 686–91. doi:10.1055/s-0031-1298334. PMID 22411724.
- ↑ U.S. Department of Agriculture (2020). "Information for Hemp Testing Laboratories". U.S. Department of Agriculture. https://www.ams.usda.gov/rules-regulations/hemp/information-laboratories.
- ↑ 27.0 27.1 Massachusetts Department of Agricultural Resources (30 April 2018). and M.G.L.-,c.,Controlled Substances Act%2C 21 U.S.C. "Interim Policy: Commercial Industrial Hemp Program" (PDF). Commonwealth of Massachusetts. https://www.mass.gov/doc/ma-hemp-program-policy/download#:~:text=Act and M.G.L.-,c.,Controlled Substances Act%2C 21 U.S.C..
- ↑ Richins, R.D.; Rodriguez-Uribe, L.; Lowe, K. et al. (2018). "Accumulation of bioactive metabolites in cultivated medical Cannabis". PLoS One 13 (7): e0201119. doi:10.1371/journal.pone.0201119. PMC PMC6056047. PMID 30036388. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6056047.
- ↑ Baron, E.P. (2018). "Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science". Headache 58 (7): 1139–86. doi:10.1111/head.13345. PMID 30152161.
- ↑ Russo, E.B. (2019). "The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No "Strain," No Gain". Frontiers in Plant Science 9: 1969. doi:10.3389/fpls.2018.01969. PMC PMC6334252. PMID 30687364. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6334252.
- ↑ Huntsman, R.J.; Tang-Wai, R.; Alcorn, J. et al. (2019). "Dosage Related Efficacy and Tolerability of Cannabidiol in Children With Treatment-Resistant Epileptic Encephalopathy: Preliminary Results of the CARE-E Study". Frontiers in Neurology 10: 716. doi:10.3389/fneur.2019.00716. PMC PMC6616248. PMID 31333569. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6616248.
- ↑ Pamplona, F.A.; da Silva, L.R.; Coan, A.C. (2018). "Potential Clinical Benefits of CBD-Rich Cannabis Extracts Over Purified CBD in Treatment-Resistant Epilepsy: Observational Data Meta-analysis". Frontiers in Neurology 9: 759. doi:10.3389/fneur.2018.00759. PMC PMC6143706. PMID 30258398. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6143706.
- ↑ Craven, C.B.; Wawryk, N.; Jiang, P. et al. (2019). "Pesticides and trace elements in cannabis: Analytical and environmental challenges and opportunities". Journal of Environmental Sciences 85: 82-93. doi:10.1016/j.jes.2019.04.028. PMID 31471034.
- ↑ Linger, P.; Müssig, J.; Fischer, H. et al. (2002). "Industrial hemp (Cannabis sativa L.) growing on heavy metal contaminated soil: Fibre quality and phytoremediation potential". Industrial Crops and Products 16 (1): 33–42. doi:10.1016/S0926-6690(02)00005-5.
- ↑ Antonkiewicz, J.; Jasiewicz, C. (2002). "The use of plants accumulating heavy metals for detoxification of chemically polluted soils". Electronic Journal of Polish Agricultural Universities 5 (1 (Environmental Development)): 1–18. http://www.ejpau.media.pl/environment/volume5/issue1/index.html.
- ↑ 36.0 36.1 Dryburgh, L.M.; Bolan, N.S.; Grof, C.P.L. et al. (2018). "Cannabis contaminants: Sources, distribution, human toxicity and pharmacologic effects". British Journal of Clinical Pharmacology 84 (11): 2468-2476. doi:10.1111/bcp.13695. PMC PMC6177718. PMID 29953631. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6177718.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added.