Difference between revisions of "Journal:Development of an integrated and comprehensive clinical trial process management system"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 49: | Line 49: | ||
The main objectives of this study are as follows. We aim (1) to develop an integrated CTMS as a dedicated database for clinical trials; (2) to achieve efficient data integration of the CTMS with hospital business systems such as the [[hospital information system]] (HIS), [[laboratory information system]] (LIS), [[Clinical data management system|clinical data repository]] (CDR), [[picture archiving and communication system]] (PACS), and [[electronic medical record]] (EMR); (3) to facilitate standardization and consistency of terminology in the development of database models and business processes; and (4) to ensure the safety and [[Information security|security]] of clinical data as well as protect patient [[Information privacy|privacy]] to comply with relevant regulations. | The main objectives of this study are as follows. We aim (1) to develop an integrated CTMS as a dedicated database for clinical trials; (2) to achieve efficient data integration of the CTMS with hospital business systems such as the [[hospital information system]] (HIS), [[laboratory information system]] (LIS), [[Clinical data management system|clinical data repository]] (CDR), [[picture archiving and communication system]] (PACS), and [[electronic medical record]] (EMR); (3) to facilitate standardization and consistency of terminology in the development of database models and business processes; and (4) to ensure the safety and [[Information security|security]] of clinical data as well as protect patient [[Information privacy|privacy]] to comply with relevant regulations. | ||
==Methods== | |||
===Overview=== | |||
The First Affiliated Hospital, Zhejiang University School of Medicine (FAHZU) has six campuses with approximately 5,000 beds. In 2020, the institution recorded over 4.2 million outpatient and emergency visits, and 236,100 discharges. As one of the pioneering and earliest-founded National Drug CTIs in China, the first batch of clinical pharmacology bases under the Ministry of Health was established in 1998. As a first-class integrated service platform for clinical research in China, it operates 24 specialized groups and depends on an internationally recognized and independent ethics examination system, and it has undertaken more than 2,000 instances of foreign and domestic clinical trials since its establishment, including both Phase I–IV drugs and [[medical device]]s. | |||
===System development process=== | |||
The development of an integrated CTMS began in March 2014, and after 18 months, the first version of the CTMS was launched in FAHZU in September 2015. The complete system development process was carried out in four stages, including two months of requirement analysis and problem collection, four months of system design, six months of system development and testing, and six months of system operation training and pilot runs. Based on the feedback from the use of the first version of CTMS and the continuous in-depth exploration of the business, the system’s functionality and user experience continue to be iteratively updated. By March 2021, the updated version 4.2.5 was launched. Through the construction of an integrated CTMS, the entire process of clinical trial data management is realized, which provides the basic conditions for the efficient, high-quality, and standardized operation of clinical trials in the hospital. | |||
====First stage==== | |||
The collection and analysis of CTMS requirements defined the existing problem set to be solved. This stage is the most critical and determines the final goal direction of the system. The collection of requirements started in March 2014, and the participants included the CTI office director, CTI office secretary, ethics committee (EC) members, EC secretary, [[principal investigator]] (PI), sub-investigator, study nurse, clinical research associate (CRA), clinical research coordinator (CRC), investigational product custodians, financial officer, quality control expert, statisticians, data manager, pharmacovigilance associate, and information technology expert. After two months of formal and informal interviews, as well as an analysis of the collection requirements, the design goal of CTMS was determined. The overall goal is for the CTMS to realize the entire process of clinical trial management, including study project approval, ethical review, subject recruitment, subject management, investigational product management, financial management, and quality management. Table 1 lists the functional requirements for realizing the entire process management of clinical trials in hospitals based on seven dimensions. These requirements were used to construct the integrated CTMS. | |||
{| | |||
| style="vertical-align:top;" | | |||
{| class="wikitable" border="1" cellpadding="5" cellspacing="0" width="70%" | |||
|- | |||
| colspan="3" style="background-color:white; padding-left:10px; padding-right:10px;" |'''Table 1.''' List of functional requirements for realizing the entire process management workflow of clinical trials in hospitals | |||
|- | |||
|- | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Number | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Requirement class | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Requirement description | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Study Project Approval Management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |The application and review of clinical trials should adopt process automation management, including process customization configuration, remote submission of project application, uploading of project materials, automatic generation of to-do tasks, timely message transmission, material annotation, and other supporting functions. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |2 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Ethical Review Management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Define the ethics committee review process and application contents (e.g., new protocols, protocol amendments, etc.). | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |3 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Subject Management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Define the subject management model to ensure that the subjects complete the visit content of each cycle in strict accordance with the research plan. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |4 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Investigational Product Management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |The investigational product should adopt the central pharmacy model to achieve closed-loop management, including receiving, warehousing, distributing, recycling, returning, disposal, and early warning. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |5 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Financial Management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Clinical trial finance requires independent accounting and management; the subjects’ diagnosis and treatment processes can be exempted from payment, and the system should automatically record costs to achieve direct settlement between the hospital and the sponsor. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |6 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Quality Management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Define the elements and content of quality management, and the quality control of related data that must be collected during the operation of the system. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |7 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Privilege Management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Define permissions and data access rules for different roles through multi-role collaboration. | |||
|- | |||
|} | |||
|} | |||
====Second stage==== | |||
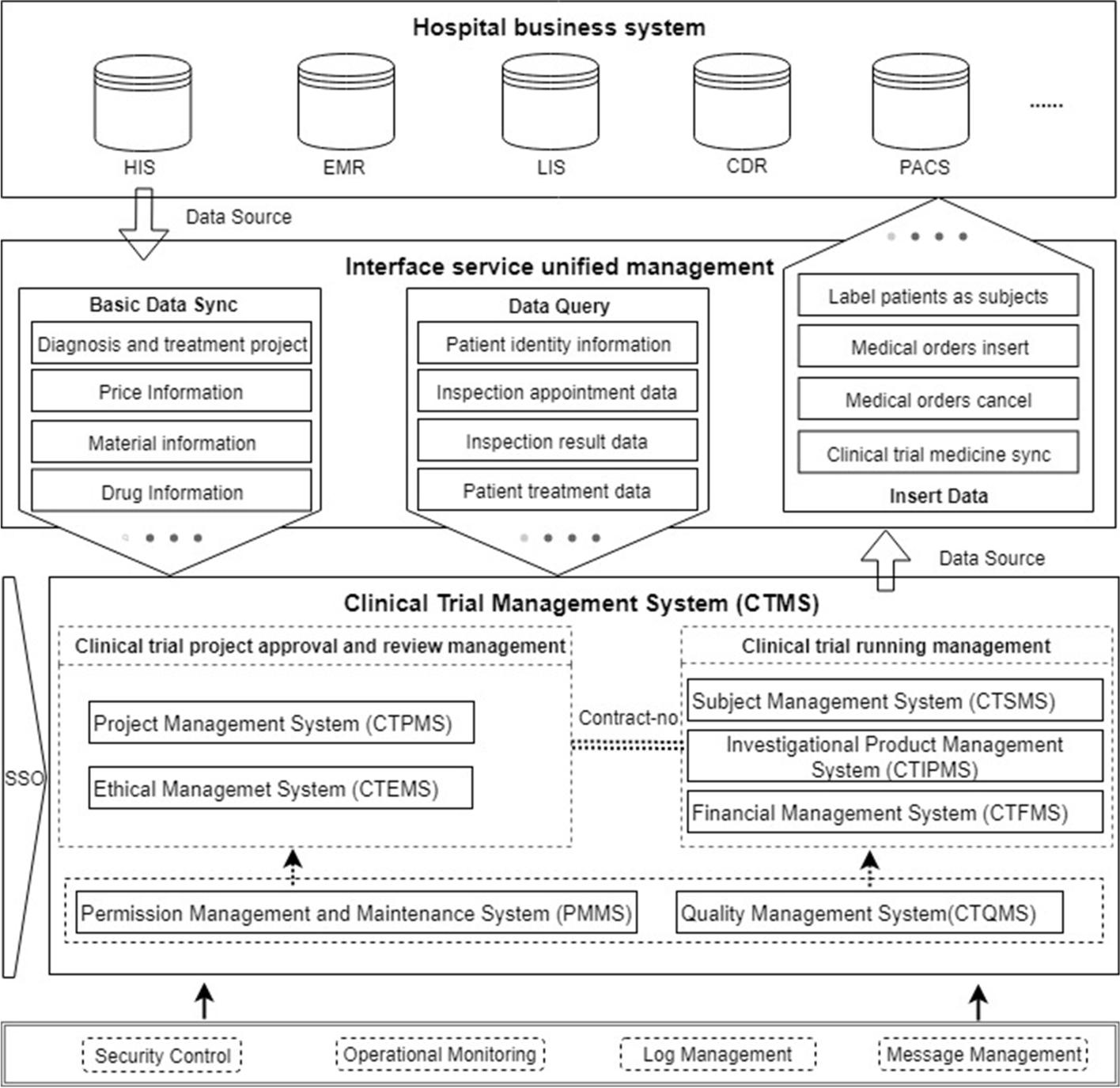
In the second stage, the CTMS design process included the definition of the system architecture and data interchange mechanisms, selection of storage media, database modeling and standardization, user interface (UI) and user experience (UX) design, and development of security and privacy policies based on analyzing and categorizing the requirements collected in the first phase, referring to good clinical practice (GCP) and the guidance documents of the National Medical Products Administration (NMPA). The functions and data required by each participant in the clinical trial are determined. Figure 1 shows the system architecture of the CTMS, which is divided into seven subsystems to meet the corresponding requirements of the first stage. Seven dimensions were put forward, including the clinical trial project management system (CTPMS), clinical trial ethical management system (CTEMS), clinical trial subject management system (CTSMS), clinical trial investigational product management system (CTIPMS), clinical trial financial management system (CTFMS), clinical trial quality management system (CTQMS), and permission management and maintenance system (PMMS). These systems include the functions of the whole-process management of clinical trials. The unified control of permissions is realized through single sign-on (SSO) between the systems. To ensure a more efficient operation of the CTMS, it is necessary to focus on the data integration mode with the hospital clinical and business systems (i.e., HIS, LIS, EMR, CDR, etc.). | |||
The unified management of interface services is defined, as shown in Fig. 1, as a process of complete data integration, which primarily involves three types of data service functions: (1) basic data synchronization, which serves as the basis for system operation; (2) real-time data query to meet the needs of subject information retrieval and clinical trial data monitoring; and (3) diagnosis and treatment data generated by the CTMS, which are transmitted to the clinical business system of the hospital to meet the continuity requirement of diagnosis and treatment of the subjects. Database modeling and standardization are performed with reference to the Clinical Data Interchange Standards Consortium (CDISC) to obtain a standard vocabulary to ensure the integrity of the data model; in terms of system security and subject privacy protection, security protection strategies and development specifications were formulated with reference to the [[Health Insurance Portability and Accountability Act]] (HIPAA) and China’s privacy protection laws. In addition, the CTMS implements fine-grained isolation and verification of permissions, and it records all events in a [[Audit trail|log]] to ensure the traceability of the data. | |||
[[File:Fig1 Shen BMCMedInfoDecMak23 23.png|900px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="900px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Fig. 1''' System architecture for the clinical trial management system (CTMS). HIS: hospital information system; EMR: electronic medical record; LIS: laboratory information system; CDR: clinical data repository; PACS: picture archiving and communication systems.</blockquote> | |||
|- | |||
|} | |||
|} | |||
====Third stage==== | |||
The third stage focused on the development and testing of the CTMS. This was divided into two steps according to the definition of the design stage. The first step realizes clinical trial approval and review management, and the developed subsystems include CTPMS, CTEMS, and PMMS, which focus on the process management of the study project application and review stage, and do not involve data integration with external systems. The second step realizes clinical trial operational management. The developed subsystems include CTSMS, CTIPMS, CTFMS, and CTQMS, which complete internal function development and data integration with external systems, and realize the entire process of data management. The CTMS was developed based on the distributed architecture of the Java programming language, and its database is Oracle Database 11 g Release 2 on a server running the Linux CentOS 7.6 operating system; data integration with external systems is managed through an interface services unified management platform. The system adopts browser/server architecture and supports current mainstream browsers, such as Google Chrome, Internet Explorer, and Firefox Browser for encrypted access via hypertext transfer protocol secure (HTTPS). System testing mainly includes verification of functional effectiveness, data security, system reliability, and the efficiency of data integration with external systems. | |||
====Fourth stage==== | |||
This stage involved CTMS deployment and system operation training. In November 2014, CTPMS, CTEMS, and PMMS were officially launched, realizing clinical trial approval and review management in FAHZU. In March 2015, the development of CTSMS, CTMMS, CTFMS, and CTQMS was completed. Considering that the clinical trial operational management involves [[data integrity]] verification and [[data integration]] with external systems, a new pilot run phase was needed. By selecting a certain number of clinical trials in Phases I–IV, the operating results were used to verify the compatibility of the system with different types of clinical trials. The CTMS pilot run system first selects Phase I clinical trials and runs a total of 20 projects. Through continuous updating of functions during operation, it can fully meet the needs of Phase I clinical trial operational management. Subsequently, it selects clinical trials in Phases II–IV. A total of 10 projects have been run, and the functions have been stable and meet the requirements of various types of clinical trials. However, training hospital staff in the use of CTMS is critical. Through a variety of methods including face-to-face training and offline data learning, all staff related to clinical trials have been trained. The first version of CTMS was fully launched in FAHZU in September 2015. | |||
FAHZU is the first hospital in China to independently develop an integrated CTMS; the system has been successfully implemented in the hospital since September 2015. As of March 2021, the hospital now runs an updated version of CTMS known as V4.2.5. The data management of the entire process of clinical trials from project approval and review management to operational management has been fully realized. The FAHZU CTMS operates independently by design as a fully functional system at the application level (not as a component of the HIS), establishes a dedicated clinical trial database at the data level, and completes data integration with external systems through a unified interface system, to achieve process continuity and data integrity. Through the construction of an integrated CTMS, the flow of multi-party collaboration tasks is optimized, and non-research matters such as finance and data processing are simplified, to effectively improve the efficiency and quality of clinical trials. The results shown below are based on the latest stable version of FAHZU CTMS, V4.2.5. | |||
===System categories and features for clinical trial=== | |||
The first level involves clinical trial project approval and review management, and its functions cover the rationality review of clinical trial study projects and related affairs management by CTI and EC. Table 2 lists the categories and features of clinical trial approval and review management, and the services provided to users through CTPMS and CTEMS. Clinical trial project approval and review management are based on multi-role collaboration, focusing on improving efficiency as the core and supporting remote project application, full electronic project approval, review of process approval documents online, aggregation of reviews into review comments, and timely generation of tasks and notification. The main features of CTPMS include project approval management, to-do task list, project list, document management, contract management, initial meeting management, investigator management, and CRC management. Through the organic combination of these features, the CTI realizes the project approval review and daily management of study projects. The main features of CTEMS include EC management, ethics review management, and ethics conference management, which manages the continuous ethics review of study projects by the EC, including initial protocol review, protocol amendment review, SAE report and review, and violation/deviation protocol review. | |||
{| | |||
| style="vertical-align:top;" | | |||
{| class="wikitable" border="1" cellpadding="5" cellspacing="0" width="70%" | |||
|- | |||
| colspan="3" style="background-color:white; padding-left:10px; padding-right:10px;" |'''Table 2.''' Categories and features for project approval and review management of clinical trials | |||
|- | |||
|- | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Category | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Features | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Description | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" rowspan="8"|Study project management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Project approval management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |CTI defines the application and review procedures for study projects according to the standard operation procedure (SOP). | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |To-do tasks list | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Lists the tasks that the user needs to complete, which are automatically generated by the system according to processes and user roles. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Project list | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Project list contains basic information about the study, controlling different viewing scopes for different roles. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Document management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Electronic management of documents, batch uploading, online review, and suggestion feedback. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Contract management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Manages the content and budget of the contract, and supervises the execution of the contract. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Initial meeting management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Records the meeting contents and participants of the initial meeting. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Investigator management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Manages investigators’ Good Clinical Practice (GCP) education, resume, etc. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Clinical research coordinator (CRC) management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Manages CRC personnel information, recruitment process, and workload reporting and review. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" rowspan="3" |Ethics management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Ethics committee (EC) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Manages the organizational structure of the EC. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Ethics review management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Manages the ethics review process, including ethical review application, formal review, study assessment, ethics conference review, approval letter generation, etc. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Ethics conference management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Manages the project review agenda, meeting attendance, voting, meeting minutes, project review results, etc. | |||
|- | |||
|} | |||
|} | |||
==Results== | |||
Revision as of 23:59, 27 June 2023
| Full article title | Development of an integrated and comprehensive clinical trial process management system |
|---|---|
| Journal | BMC Medical Informatics and Decision Making |
| Author(s) | Shen, Liang; Zhai, You; Pan, AXiang; Zhao, Qingwei; Zhou, Min; Lio, Jian |
| Author affiliation(s) | Zhejiang University School of Medicine |
| Primary contact | Email: minzhou at zju dot edu dot cn |
| Year published | 2023 |
| Volume and issue | 23 |
| Article # | 61 |
| DOI | 10.1186/s12911-023-02158-8 |
| ISSN | 1472-6947 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-023-02158-8 |
| Download | https://bmcmedinformdecismak.biomedcentral.com/counter/pdf/10.1186/s12911-023-02158-8.pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Background: The process of initiating and completing clinical drug trials in hospital settings is highly complex, with numerous institutional, technical, and record-keeping barriers. In this study, we independently developed an integrated clinical trial management system (CTMS) designed to comprehensively optimize the process management of clinical trials. The CTMS includes system development methods, efficient integration with external business systems, terminology, and standardization protocols, as well as data security and privacy protection.
Methods: The development process proceeded through four stages, including demand analysis and problem collection, system design, system development and testing, system trial operation, and training the whole hospital to operate the system. The integrated CTMS comprises three modules: project approval and review management, clinical trial operations management, and background management modules. These are divided into seven subsystems and 59 internal processes, realizing all the functions necessary to comprehensively perform the process management of clinical trials. Efficient data integration is realized through extract-transform-load (ETL), message queue, and remote procedure call services with external systems such as the hospital information system (HIS), laboratory information system (LIS), electronic medical record (EMR), and clinical data repository (CDR). Data security is ensured by adopting corresponding policies for data storage and data access. Privacy protection complies with laws and regulations and de-identifies sensitive patient information.
Results: The integrated CTMS was successfully developed in September 2015 and updated to version 4.2.5 in March 2021. During this period, 1,388 study projects were accepted, 43,051 electronic data files stored, and 12,144 subjects recruited in the First Affiliated Hospital, Zhejiang University School of Medicine.
Conclusion: The developed integrated CTMS realizes the data management of the entire clinical trials process, providing basic conditions for the efficient, high-quality, and standardized operation of clinical trials.
Keywords: clinical trial, clinical trial management system (CTMS), information technology, integrated management system, medical informatics
Background
Clinical trials of drugs are an important stage in drug research and development and are means to improve the development of medical science and technology. [1,2,3,4,5]. Standardizing the management of clinical research is key to simplifying the research process, improving quality, and ensuring the accuracy, reliability, and integrity of the results, thereby shortening the development cycle of new drugs and accelerating the process of drug registration. [6]
In China, each hospital has a dedicated clinical trial management department, called a clinical trial institution (CTI), responsible for the administrative management of clinical trial operation in the hospital. This involves determining the project leader, formulating research plans with sponsors, reviewing the plans with the ethics committee, signing research contracts, screening and enrolling subjects, originating records of trials, receiving drugs, distributing and recovering, processing or reporting adverse events (AE) or serious adverse events (SAE), managing in-hospital quality, accepting supervision and inspection, summarizing, and filing. The traditional management mode is based on paper documents and involves considerable manual labor, which often leads to untimely information updates, ineffective management, and unverifiable quality.
Today, the rapid development of internet technology has profoundly impacted the mode of clinical trial management. Diverse companies have developed a series of network management systems for clinical trials. [7, 8] Most of these systems have been developed based on the research and development (R&D) requirements of pharmaceutical companies. Clinical trials are managed in a networked manner, which only handles project-related data and excludes the process management links of the clinical trial organization. These commercialized clinical trial management systems (CTMSs) provide comprehensive clinical trial management services, supporting all types of clinical trials, from Phase I to Phase III, from a trial conducted in a single research center to multinational clinical trials; thus, they improve the quality of clinical trials and the efficiency of data management. However, from the perspective of drug clinical trial institutions, this type of CTMS cannot satisfy the practical requirements for real-time and effective management of all clinical trials carried out by these institutions. Hence, a new CTMS has been developed as an institutional management model in China. Its development and application are still in their infancy. The CTMS also faces certain incompatibility issues with the hospital database. It is difficult to achieve data sharing and docking. Moreover, the management method is still relatively primitive. The degree of digitalization, networking, and standardization of clinical trials is relatively low, and it is practically impossible for institutions to effectively manage clinical trials.
In recent years, owing to the increase in clinical trial projects in our hospital, professional and standardized information systems were urgently needed to assist in the management of clinical trials throughout the hospital. At present, the need for a centralized clinical trial project management platform reliant on the clinical data system of the hospital itself is intensifying. Based on the local area network (LAN) security architecture of our hospital, we have constructed a CTMS, which organically integrates the clinical trial organization management office, ethics committee, clinical trial center pharmacy, and clinical professional departments. The management system covers the entire process of clinical trials, including trial project establishment, ethical review, signing of agreements, trial implementation, trial conclusion, sponsor management, subject management, follow-up management, and centralized management of pharmacies supporting medication trials. The personnel involved include clinical departments, institutional management offices, central pharmacies, and ethics committees. With the help of the information system, a large number of personnel and complicated work processes are now more organically integrated to realize information sharing and collaborative work. With the help of this CTMS, the implementation of clinical trials can be standardized and the entire process can be traceable.
As a site of clinical trials, hospital management involves multi-party collaboration, as well as study project management, subject management, investigational product management, quality control, financial management, and other complex business processes. Hospitals must develop dedicated systems to assist the entire process management of clinical trials to ensure their efficient and high-quality operation.
The main objectives of this study are as follows. We aim (1) to develop an integrated CTMS as a dedicated database for clinical trials; (2) to achieve efficient data integration of the CTMS with hospital business systems such as the hospital information system (HIS), laboratory information system (LIS), clinical data repository (CDR), picture archiving and communication system (PACS), and electronic medical record (EMR); (3) to facilitate standardization and consistency of terminology in the development of database models and business processes; and (4) to ensure the safety and security of clinical data as well as protect patient privacy to comply with relevant regulations.
Methods
Overview
The First Affiliated Hospital, Zhejiang University School of Medicine (FAHZU) has six campuses with approximately 5,000 beds. In 2020, the institution recorded over 4.2 million outpatient and emergency visits, and 236,100 discharges. As one of the pioneering and earliest-founded National Drug CTIs in China, the first batch of clinical pharmacology bases under the Ministry of Health was established in 1998. As a first-class integrated service platform for clinical research in China, it operates 24 specialized groups and depends on an internationally recognized and independent ethics examination system, and it has undertaken more than 2,000 instances of foreign and domestic clinical trials since its establishment, including both Phase I–IV drugs and medical devices.
System development process
The development of an integrated CTMS began in March 2014, and after 18 months, the first version of the CTMS was launched in FAHZU in September 2015. The complete system development process was carried out in four stages, including two months of requirement analysis and problem collection, four months of system design, six months of system development and testing, and six months of system operation training and pilot runs. Based on the feedback from the use of the first version of CTMS and the continuous in-depth exploration of the business, the system’s functionality and user experience continue to be iteratively updated. By March 2021, the updated version 4.2.5 was launched. Through the construction of an integrated CTMS, the entire process of clinical trial data management is realized, which provides the basic conditions for the efficient, high-quality, and standardized operation of clinical trials in the hospital.
First stage
The collection and analysis of CTMS requirements defined the existing problem set to be solved. This stage is the most critical and determines the final goal direction of the system. The collection of requirements started in March 2014, and the participants included the CTI office director, CTI office secretary, ethics committee (EC) members, EC secretary, principal investigator (PI), sub-investigator, study nurse, clinical research associate (CRA), clinical research coordinator (CRC), investigational product custodians, financial officer, quality control expert, statisticians, data manager, pharmacovigilance associate, and information technology expert. After two months of formal and informal interviews, as well as an analysis of the collection requirements, the design goal of CTMS was determined. The overall goal is for the CTMS to realize the entire process of clinical trial management, including study project approval, ethical review, subject recruitment, subject management, investigational product management, financial management, and quality management. Table 1 lists the functional requirements for realizing the entire process management of clinical trials in hospitals based on seven dimensions. These requirements were used to construct the integrated CTMS.
| |||||||||||||||||||||||||||
Second stage
In the second stage, the CTMS design process included the definition of the system architecture and data interchange mechanisms, selection of storage media, database modeling and standardization, user interface (UI) and user experience (UX) design, and development of security and privacy policies based on analyzing and categorizing the requirements collected in the first phase, referring to good clinical practice (GCP) and the guidance documents of the National Medical Products Administration (NMPA). The functions and data required by each participant in the clinical trial are determined. Figure 1 shows the system architecture of the CTMS, which is divided into seven subsystems to meet the corresponding requirements of the first stage. Seven dimensions were put forward, including the clinical trial project management system (CTPMS), clinical trial ethical management system (CTEMS), clinical trial subject management system (CTSMS), clinical trial investigational product management system (CTIPMS), clinical trial financial management system (CTFMS), clinical trial quality management system (CTQMS), and permission management and maintenance system (PMMS). These systems include the functions of the whole-process management of clinical trials. The unified control of permissions is realized through single sign-on (SSO) between the systems. To ensure a more efficient operation of the CTMS, it is necessary to focus on the data integration mode with the hospital clinical and business systems (i.e., HIS, LIS, EMR, CDR, etc.).
The unified management of interface services is defined, as shown in Fig. 1, as a process of complete data integration, which primarily involves three types of data service functions: (1) basic data synchronization, which serves as the basis for system operation; (2) real-time data query to meet the needs of subject information retrieval and clinical trial data monitoring; and (3) diagnosis and treatment data generated by the CTMS, which are transmitted to the clinical business system of the hospital to meet the continuity requirement of diagnosis and treatment of the subjects. Database modeling and standardization are performed with reference to the Clinical Data Interchange Standards Consortium (CDISC) to obtain a standard vocabulary to ensure the integrity of the data model; in terms of system security and subject privacy protection, security protection strategies and development specifications were formulated with reference to the Health Insurance Portability and Accountability Act (HIPAA) and China’s privacy protection laws. In addition, the CTMS implements fine-grained isolation and verification of permissions, and it records all events in a log to ensure the traceability of the data.
|
Third stage
The third stage focused on the development and testing of the CTMS. This was divided into two steps according to the definition of the design stage. The first step realizes clinical trial approval and review management, and the developed subsystems include CTPMS, CTEMS, and PMMS, which focus on the process management of the study project application and review stage, and do not involve data integration with external systems. The second step realizes clinical trial operational management. The developed subsystems include CTSMS, CTIPMS, CTFMS, and CTQMS, which complete internal function development and data integration with external systems, and realize the entire process of data management. The CTMS was developed based on the distributed architecture of the Java programming language, and its database is Oracle Database 11 g Release 2 on a server running the Linux CentOS 7.6 operating system; data integration with external systems is managed through an interface services unified management platform. The system adopts browser/server architecture and supports current mainstream browsers, such as Google Chrome, Internet Explorer, and Firefox Browser for encrypted access via hypertext transfer protocol secure (HTTPS). System testing mainly includes verification of functional effectiveness, data security, system reliability, and the efficiency of data integration with external systems.
Fourth stage
This stage involved CTMS deployment and system operation training. In November 2014, CTPMS, CTEMS, and PMMS were officially launched, realizing clinical trial approval and review management in FAHZU. In March 2015, the development of CTSMS, CTMMS, CTFMS, and CTQMS was completed. Considering that the clinical trial operational management involves data integrity verification and data integration with external systems, a new pilot run phase was needed. By selecting a certain number of clinical trials in Phases I–IV, the operating results were used to verify the compatibility of the system with different types of clinical trials. The CTMS pilot run system first selects Phase I clinical trials and runs a total of 20 projects. Through continuous updating of functions during operation, it can fully meet the needs of Phase I clinical trial operational management. Subsequently, it selects clinical trials in Phases II–IV. A total of 10 projects have been run, and the functions have been stable and meet the requirements of various types of clinical trials. However, training hospital staff in the use of CTMS is critical. Through a variety of methods including face-to-face training and offline data learning, all staff related to clinical trials have been trained. The first version of CTMS was fully launched in FAHZU in September 2015.
FAHZU is the first hospital in China to independently develop an integrated CTMS; the system has been successfully implemented in the hospital since September 2015. As of March 2021, the hospital now runs an updated version of CTMS known as V4.2.5. The data management of the entire process of clinical trials from project approval and review management to operational management has been fully realized. The FAHZU CTMS operates independently by design as a fully functional system at the application level (not as a component of the HIS), establishes a dedicated clinical trial database at the data level, and completes data integration with external systems through a unified interface system, to achieve process continuity and data integrity. Through the construction of an integrated CTMS, the flow of multi-party collaboration tasks is optimized, and non-research matters such as finance and data processing are simplified, to effectively improve the efficiency and quality of clinical trials. The results shown below are based on the latest stable version of FAHZU CTMS, V4.2.5.
System categories and features for clinical trial
The first level involves clinical trial project approval and review management, and its functions cover the rationality review of clinical trial study projects and related affairs management by CTI and EC. Table 2 lists the categories and features of clinical trial approval and review management, and the services provided to users through CTPMS and CTEMS. Clinical trial project approval and review management are based on multi-role collaboration, focusing on improving efficiency as the core and supporting remote project application, full electronic project approval, review of process approval documents online, aggregation of reviews into review comments, and timely generation of tasks and notification. The main features of CTPMS include project approval management, to-do task list, project list, document management, contract management, initial meeting management, investigator management, and CRC management. Through the organic combination of these features, the CTI realizes the project approval review and daily management of study projects. The main features of CTEMS include EC management, ethics review management, and ethics conference management, which manages the continuous ethics review of study projects by the EC, including initial protocol review, protocol amendment review, SAE report and review, and violation/deviation protocol review.
| ||||||||||||||||||||||||||||||
Results
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation, grammar, and punctuation. In some cases important information was missing from the references, and that information was added.