Difference between revisions of "Journal:Digitalization of calibration data management in the pharmaceutical industry using a multitenant platform"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 91: | Line 91: | ||
Due to the complexity of the MI and diversity of the organizations involved, the progress of digitalization has been slow, and in many cases data are still being managed in paper or paper-on-glass formats, requiring human interpretation, e.g., in the case of documenting calibrations in calibration certificates. [15] For field calibrations, digitalized solutions have become more common, as the costs of calibrations are proportional to the efficiency of the processes. However, these solutions tend to be instrument- and system-provider-specific, and due to competition, the willingness of the system providers to collaborate by harmonizing their systems and making them more open has been low. In addition, it is common that larger industrial companies cooperate with several instrument manufacturers and service providers. Consequently, in the worst case for the instrument owner, the data exchange from different organizations has been scattered in several systems using different formats. Because of this, human resources relative to the number of annual calibrations have been tied up in calibration management alone to ensure compliance with quality management regulations, meaning that it has been a significant expense. | Due to the complexity of the MI and diversity of the organizations involved, the progress of digitalization has been slow, and in many cases data are still being managed in paper or paper-on-glass formats, requiring human interpretation, e.g., in the case of documenting calibrations in calibration certificates. [15] For field calibrations, digitalized solutions have become more common, as the costs of calibrations are proportional to the efficiency of the processes. However, these solutions tend to be instrument- and system-provider-specific, and due to competition, the willingness of the system providers to collaborate by harmonizing their systems and making them more open has been low. In addition, it is common that larger industrial companies cooperate with several instrument manufacturers and service providers. Consequently, in the worst case for the instrument owner, the data exchange from different organizations has been scattered in several systems using different formats. Because of this, human resources relative to the number of annual calibrations have been tied up in calibration management alone to ensure compliance with quality management regulations, meaning that it has been a significant expense. | ||
As digitalization in the industry and other sectors is progressing, and the collection and use of data is growing rapidly, the topics concerning [[data quality]] and trustworthiness become increasingly important, which is driving the research and development efforts in digitalization within the metrology community. [7,18] The ongoing efforts include the definition and development of digital formats for presenting metrological information, e.g., data models for presenting metrologically relevant data as semantic metadata [19,20,21], or formats for digital calibration certificates. [15,22,23,24] For this work, the exchange of DCCs was tested using the DCC format originally defined by the Physikalisch-Technische Bundesanstalt (PTB) and further developed in EMPIR SmartCom and Gemimeg research projects. [4,5,25,26,27,28] | As digitalization in the industry and other sectors is progressing, and the collection and use of data is growing rapidly, the topics concerning [[data quality]] and trustworthiness become increasingly important, which is driving the research and development efforts in digitalization within the metrology community. [7,18] The ongoing efforts include the definition and development of digital formats for presenting metrological information, e.g., data models for presenting metrologically relevant data as semantic [[metadata]] [19,20,21], or formats for digital calibration certificates. [15,22,23,24] For this work, the exchange of DCCs was tested using the DCC format originally defined by the Physikalisch-Technische Bundesanstalt (PTB) and further developed in EMPIR SmartCom and Gemimeg research projects. [4,5,25,26,27,28] | ||
Digitization of the data formats sets new requirements for the development of the infrastructure, as its operation has been based on mutual recognition and trust between organizations. [16] Transition to a digital environment also means that this trust needs to be established digitally to enable its representation in a machine-understandable format. [29,30,31] For this purpose, [[Information security|data security]] and cryptographical solutions, e.g., the use of digital signatures, are relevant. [15,17] | Digitization of the data formats sets new requirements for the development of the infrastructure, as its operation has been based on mutual recognition and trust between organizations. [16] Transition to a digital environment also means that this trust needs to be established digitally to enable its representation in a machine-understandable format. [29,30,31] For this purpose, [[Information security|data security]] and cryptographical solutions, e.g., the use of digital signatures, are relevant. [15,17] | ||
| Line 104: | Line 104: | ||
In terms of quality management, the effects of Industry 4.0 are prominent in the digitalization of methodologies such as total quality management (TQM) and the use of IoT technologies in quality management operations and processes. [39] In some cases, the advancements in IoT technologies have also led to situations where traditional quality management is not in alignment with the requirements of IoT devices. [40] | In terms of quality management, the effects of Industry 4.0 are prominent in the digitalization of methodologies such as total quality management (TQM) and the use of IoT technologies in quality management operations and processes. [39] In some cases, the advancements in IoT technologies have also led to situations where traditional quality management is not in alignment with the requirements of IoT devices. [40] | ||
In the process and pharmaceutical industries specifically, the benefits of IoT and Industry 4.0 can be seen as being the same as in other manufacturing industries. [41,42] However, in the pharmaceutical industry, digitalization has been slowed due to the interdependence of processes and strict regulations requiring, e.g., comprehensive [[Software verification and validation|validation]] of newly developed systems and software, due to which the investments in Industry 4.0 solutions can be both risky and expensive. [43,44,45] In a survey by Reinhardt ''et al.'', the main areas of focus for Industry 4.0 adoption in the pharmaceutical industry were optimizing processes, monitoring production performance, maintaining regulatory compliance, and minimizing production downtime. [46] | |||
===Requirements for the calibration data management and data formats=== | |||
Section 7.6 of the [[ISO 9000|ISO 9001]] standard for QMSs contains the general requirements for the handling of measuring and monitoring equipment. [47] The standard requires that the equipment used for quantitative measurements must be calibrated periodically, for which the instrument owner must define and document an operational procedure. | |||
For the process and pharmaceutical industry, there are legislative requirements for the federal legislations, e.g., pharmacopeias and good practices (GxP) such as [[good manufacturing practice]] (GMP) and good laboratory practices (GLP). | |||
The main standard defining the requirements for calibration data management and certificate formats for accredited laboratories is the [[ISO/IEC 17025]] standard on general requirements for the competence of testing and calibration laboratories. [48] This standard, accompanied by other more general standards such as ISO 9001, is used as the basis for the accreditation of calibration laboratories. [49] The requirements for the contents of a calibration certificate are defined in Section 7.8 of the ISO/IEC 17025 standard. | |||
====General guidelines and good practices==== | |||
In addition to international standards and regional legislations, there also exist several non-obligatory recommendations in the form of good practices or guidelines by different organizations, which have become widely used and unofficially agreed ''de facto'' standards for the industry. These ''de facto'' standards can be guidelines by NMIs or regional metrology organizations (RMO) on calibration procedures for specific instrument types, guidelines by societies and associations of the experts in the field [50,51], or guides and whitepapers by companies, e.g., instrument manufacturers, which are typically based on the relevant legislative requirements. | |||
====Mass as an example measurand==== | |||
The differences that the standards, regulations, and guidelines of the different organizations may have can be pointed out by examining a singular physical quantity and the respective measurement methodologies. Harmonization is beneficial, especially given that differences in the calibration procedures can result in differences, e.g., in uncertainty evaluations. [52] Good examples of the harmonization of practices on a global level are the most recent revisions of the regulations and guidelines for mass and weighing, e.g., non-automatic weighing instruments. | |||
Standards covering the use of non-automatic weighing instruments include the European EN 45501:2015 ''Metrological aspects of non-automatic weighing instruments'' [53] and the American NIST Handbook 44 ''Specifications, Tolerances, and Other Technical Requirements for Weighing and Measuring Devices''. [54] | |||
Regional guidelines for the calibration procedures for weighing instruments are published by the RMOs. In the case of non-automatic weighing instruments, these guidelines include, for example, Euramet Calibration Guideline No. 18 ''Guidelines on the Calibration of Non-Automatic Weighing Instruments'' [55], ASTM E898 ''Standard practice for calibration of non-automatic weighing instruments'' [56], SIM MWG7 ''Guidelines on the calibration of non-automatic weighing instruments'' [57], and JJF 1847 ''Calibration Specifications for Electronic Balances''. [58] Harmonization of these guidelines has been based on the other organizations adapting their guidelines to be commensurate with the EURAMET CG-18, which has also become better known in the Asian region, allowing the requirements to become a global ''de facto'' standard. [11] | |||
At the industrial level, the requirements for the weighing instruments and other measurement instruments are defined in the regional or national regulations for pharmaceutical products. In the ''US Pharmacopeia'' (''USP''), the requirements for balances are covered in General Chapter 41 “Balances,” [9] with supporting guidance given in General Chapter 1251 “Weighing on an Analytical Balance.” [59] Respectively, in the ''European Pharmacopoeia'', the requirements for balances are covered in General Chapter 2.1.7 “Balances for analytical purposes.” [10] After the latest revision of the ''European Pharmacopoeia'', the requirements are commensurate with the ''USP'', which has been a welcome advancement towards the harmonization of regulations. [11] | |||
====Requirements for data integrity==== | |||
One of the topics where improvements are pursued by investing in digitalization in the pharmaceutical industry is data integrity. The basis for data integrity regulation compliance is typically referred to as the ALCOA+ principle [60,61], the definition of which is presented in Table 1. | |||
{| | |||
| style="vertical-align:top;" | | |||
{| class="wikitable" border="1" cellpadding="5" cellspacing="0" width="80%" | |||
|- | |||
| colspan="3" style="background-color:white; padding-left:10px; padding-right:10px;" |'''Table 1.''' The definition of the ALCOA+ principle. [60,61] | |||
|- | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Symbol | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Meaning | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;" |Definition | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |A | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Attributable | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |It must be clear when an action was taken and by whom, as well as where the data came from, e.g., the systems, devices, or instruments used. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |L | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Legible and intelligible | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |The data and records must be comprehensible regardless of the record types. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |C | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Contemporaneous | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Recordings must take place at the time of the action or observation, and a timestamp must be included. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |O | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Original | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |If a record is copied, the original record must be distinguishable, and the copies must be verified as “true copies.” Especially for electronic records, all relevant metadata must also be considered. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |A | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Accurate | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |All instruments used for collecting data must be accurate and controlled, e.g., calibrated, and any corrections or changes that need to be made in the records must be documented as amendments. | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |+ | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Available<br /> <br />Complete<br /> <br />Consistent<br /> <br />Enduring | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |All records must be accessible and available for audits over their lifetime.<br /> <br />All relevant data and metadata must be included in the records and must not be omitted or deleted.<br /> <br />The timestamps in the records of actions and observations must be consistent with the order of the steps in the operation procedure and be based on a common time reference.<br /> <br />The media used to store the records must be authorized and robust to ensure the preservation of the record over its lifetime. | |||
|- | |||
|} | |||
|} | |||
Regulatory requirements for data integrity in manufacturing are either defined as a part of the GMPs and GLPs or in separate regulations such as the [[Food and Drug Administration|US Food and Drug Administration]]'s (FDA's) [[21 CFR Part 11|Title 21 Code of Federal Regulations Part 11]] (21 CFR Part 11) [62] or the Medicines & Healthcare Products Regulatory Agency's (MHRA's) Data Integrity Guidance and Definitions. [63] An example of a ''de facto'' standard on data integrity is the ''ISPE GAMP Guide: Records & Data Integrity'' [12], where data integrity requirements defined in the regulations are approached in a more practical way. | |||
Data integrity is also covered, although to a lesser extent, in Section 7.11 of ISO/IEC 17025, where requirements for data management systems used by calibration laboratories for collecting, processing, recording, reporting, storing, or retrieving data are defined. [48] However, as the requirements for the calibration certificates focus on the content that must be included, regardless of whether the certificate is a physical or electronic document, the standard does not specify detailed requirements or methods for securing a digital file. | |||
====Harmonization challenges for developing digital solutions==== | |||
The challenge in developing harmonized and interoperable formats and data exchange between the calibration service providers and the customers is that the requirements for the process and its documentation depend on the roles of the organizations in a calibration chain. For the NMI's and accredited laboratories, the calibration practices are mostly defined in the ISO/IEC 17025 standard and other requirements that are mandated as a part of the accreditation. | |||
However, a significant amount of the calibrations performed in the industry are not done according to the ISO/IEC 17025 standard, mostly due to costs. This includes field calibrations that are performed on-site, as this is far more cost-efficient in comparison to having all the process-controlling instruments calibrated at a separate laboratory. For these calibrations, the requirements are defined in the quality management regulations and legislation of the national and regional authorities. Compared to ISO/IEC 17025, which specifically focuses on calibrations, legislative requirements focus on the QMS as a whole. The main principles regarding the calibrations of the instruments are no different, but the context and reasoning behind them is. | |||
For NMIs and accredited laboratories, calibrations are a service that they provide for their customers, and they are regulated and audited to ensure that their processes are up to the level that is required; this is why the requirements are very specific. On the other hand, in the industry’s end of the calibration chain, the regulations are not as specifically defined in all cases. For example, instead of requiring, e.g., calibration processes to follow a specifically defined procedure, companies are mandated to have written instructions for the processes, meaning that some of the details are left open for companies to define themselves. Due to this openness, there are some guidelines and good practices that have been adopted as ''de facto'' standards for calibration procedures. To shortly summarize the differences between the regulations for accredited calibrations and field calibrations, accredited calibrations are focused on an individual instrument, whereas field calibrations consider the instrument a part of a process line. | |||
The effects of these differences in the requirements can be noticed when comparing a typical format for a field calibration certificate to a certificate issued by an accredited laboratory. The field calibration certificates must include information such as the identification of the position or location where the instrument has been used in a particular process line—which is relevant information for the quality assurance of that process line—and a calibration due date based on the calibration interval specified in the manufacturing company’s requirements. | |||
For the calibration data, the main difference is that the calibration data have been presented in a different way. The standards for the accredited laboratories require that the calibration results are presented, including the measurement uncertainties of the measurement points, whereas in the regulations for the industry, it is relatively common that the deviations or measurement errors are required. Another example of these kinds of differences includes requirements for the use of different units in different parts of the world. | |||
In addition to the variances in requirements, some challenges for the digitalization of the processes arise from the way in which the requirements have been defined in the standards and legislation, as the current formats are written for human interpretation. The [[International Electrotechnical Commission]] (IEC) has presented a utility model for SMART Standards. [64] The model defines the following levels for the digitization degree of a standard: | |||
* Level 0: Paper format - This format is not suitable for direct automatic processing or usage. | |||
* Level 1: Digital format (e.g., PDF) - This format allows automatic management and display of the document. | |||
* Level 2: Machine-readable format - The structure of the document can be digitized, and certain granular content can be exported (chapters, graphics, definitions, etc.). Content and presentation are separated. | |||
* Level 3: Machine-readable and -executable content - All essential granular information units can be clearly identified, and their reciprocal relationships can be recorded and made available for further processing or partial implementation. | |||
* Level 4: Machine-interpretable content - The information in a standard is linked with implementation and usage information in such a way that it is implemented by machines directly or interpreted and combined with other information sources so that complex actions and decision-making processes take place automatically. | |||
The German Institute for Standardisation (DIN) and German Association for Electrical, Electronic & Information Technologies (DKE) have presented an additional level that goes beyond Level 4 in the IEC model [64]: | |||
* Level 5: Machine-controllable content - The content of a standard can be amended by machines working unassisted and adopted by automated (distributed) decision-making processes. The content adopted in this way is automatically reviewed and published via the publication channels of the standardization organizations. | |||
The model aptly emphasizes the difference among machine-readable, -executable, -interpretable, and -controllable formats. Ideally, for the development of digital calibration management processes, the requirements would have to be machine-interpretable, enabling requirement-specific data and criteria such as allowance limits to be directly interpreted by the management systems as process parameters, making further automation of the processes management possible. Currently, when the requirements are not yet available in such a format, this kind of specific information must be included in the systems through human interpretation, which means that revisions of the requirements will require updating of the systems accordingly. | |||
==Materials and methods== | |||
===Studied cases for the proof of concept=== | |||
In the proof of concept (POC), the focus of the work was to harmonize the data exchange between the parties of a calibration chain to enable automation of the data management processes in the receiving end, thus eliminating the need for manual work to interpret and input the data into calibration management systems (CMS) or [[enterprise resource planning]] (ERP) systems, making the processes more efficient and improving data integrity by reducing the chance of input errors. To achieve these goals, use of a multitenant platform was chosen as the basis for defining the data exchange processes and methods. | |||
The DCC formats used in the POCs were the DCC XML schema versions 2.4.0<ref name="PTBDCC240">{{cite web |url=https://www.ptb.de/dcc/v2.4.0/dcc.xsd |title=xmlns:dcc schema version 2.4.0 |author=Physikalisch-Technische Bundesanstalt |date=2019 |accessdate=06 June 2022}}</ref> in Case 1 and 3.0.0-rc2<ref name="PTBDCC300RC2">{{cite web |url=https://www.ptb.de/dcc/v3.0.0-rc.2/dcc.xsd |title=xmlns:dcc schema version 3.0.0-rc.2 |author=Physikalisch-Technische Bundesanstalt |date=2019 |accessdate=06 June 2022}}</ref> in Case 2. In both cases, the means of using the DCC schema were agreed upon based on the requirements and needs of the participating organizations representing the calibration customer. The DCCs used in the testing were developed based on examples of existing calibration certificates. | |||
====Case 1==== | |||
In Case 1, the exchange and handling of DCCs was tested by mimicking a realistic calibration chain from a national measurement standard of an NMI to a working standard of a pharmaceutical manufacturing company. The main objectives for the POC were achieving an efficient and easy-to-use method for managing calibrations and the related data exchange between organizations. The physical quantities for which the harmonized file formats were agreed upon within the consortium were temperature and pressure, as the working standard in question is capable of measuring both quantities. In addition to Aalto University, the organizations involved were: | |||
* VTT MIKES, the Finnish NMI; | |||
* Vaisala Oyj, an instrument manufacturer and a provider of calibration services, with an accredited laboratory; | |||
* Orion Oyj, a pharmaceutical company; and | |||
* Beamex Oy Ab, a field calibration instrument and CMS provider. | |||
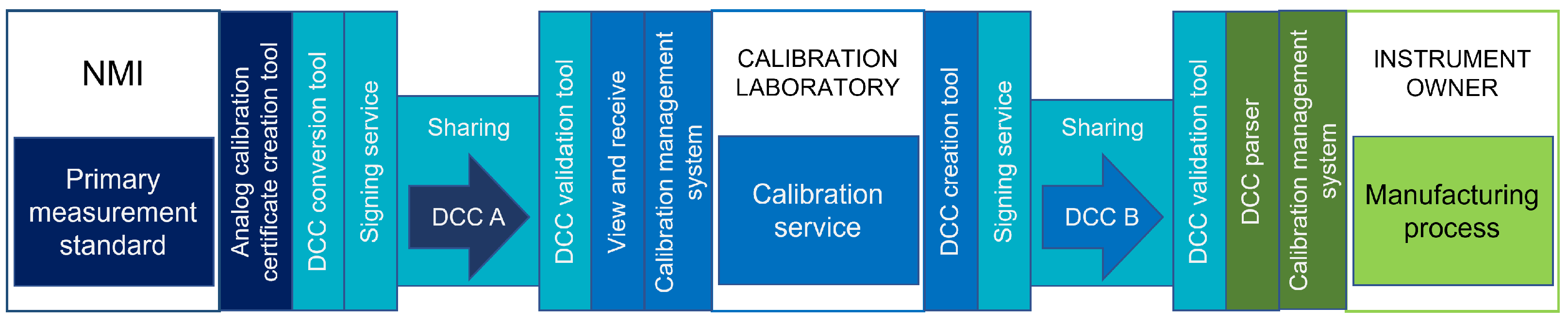
Figure 3 shows the POC setting, where different colors highlight the systems and operations of the organizations involved. | |||
[[File:Fig3 Mustapää ApplSciences22 12-15.png|1000px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="1000px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Figure 3.''' An illustration of the POC implementation. VTT MIKES is represented in dark blue, Vaisala in blue, Aalto University in turquoise, Beamex in dark green, and Orion in light green.</blockquote> | |||
|- | |||
|} | |||
|} | |||
==References== | ==References== | ||
| Line 112: | Line 225: | ||
==Notes== | ==Notes== | ||
This presentation is faithful to the original, with only a few minor changes to presentation. Grammar was tweaked significantly to improve readability. The PMCID and DOI were also added when they were missing from the original reference. | This presentation is faithful to the original, with only a few minor changes to presentation. Grammar was tweaked significantly to improve readability. The PMCID and DOI were also added when they were missing from the original reference. The links to the DCC XML schemas of the original article were turned into proper references for this version. | ||
<!--Place all category tags here--> | <!--Place all category tags here--> | ||
Revision as of 16:48, 1 March 2023
| Full article title | Digitalization of calibration data management in the pharmaceutical industry using a multitenant platform |
|---|---|
| Journal | Applied Sciences |
| Author(s) | Mustapää, Tuukka; Nummiluikki, Juho; Viitala, Raine |
| Author affiliation(s) | Aalto University, Beamex Oy Ab |
| Primary contact | Email: tuukka dot mustapaa at aalto dot fi |
| Editors | Mendyk, Aleksander |
| Year published | 2022 |
| Volume and issue | 12(15) |
| Article # | 7531 |
| DOI | 10.3390/app12157531 |
| ISSN | 2076-3417 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.mdpi.com/2076-3417/12/15/7531 |
| Download | https://www.mdpi.com/2076-3417/12/15/7531/pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
The global quality infrastructure (QI) has been established and is maintained to ensure the safety of products and services for their users. One of the cornerstones of the QI is metrology, i.e., the science of measurement, as quality management systems (QMS) commonly rely on measurements for evaluating quality. For this reason, calibration procedures and management of the data related to them are of the utmost importance for quality management in the process industry, made a particularly high priority by regulatory authorities. To overcome the relatively low level of digitalization in metrology, machine-interpretable data formats such as digital calibration certificates (DCC) are being developed.
In this paper, we analyze the current calibration processes in the pharmaceutical industry, and the requirements defined for them in the relevant standards and regulations. For digitalizing the calibration-related data exchange, a multitenant cloud- and platform-based method is presented. To test and validate the approach, a proof of concept (POC) implementation of the platform is developed with a focus on ease and cost-efficiency of deployment and use, while also ensuring the preservation of traceability and data integrity. The POC is based on two industrial use cases involving organizations with different roles in the metrology infrastructure. From testing this POC, the presented approach proves to be an efficient method for organizing the calibration data exchange in industrial use.
Keywords: digitalization, quality management, pharmaceutical industry, digital calibration certificate, metrology, traceability, platform
Introduction
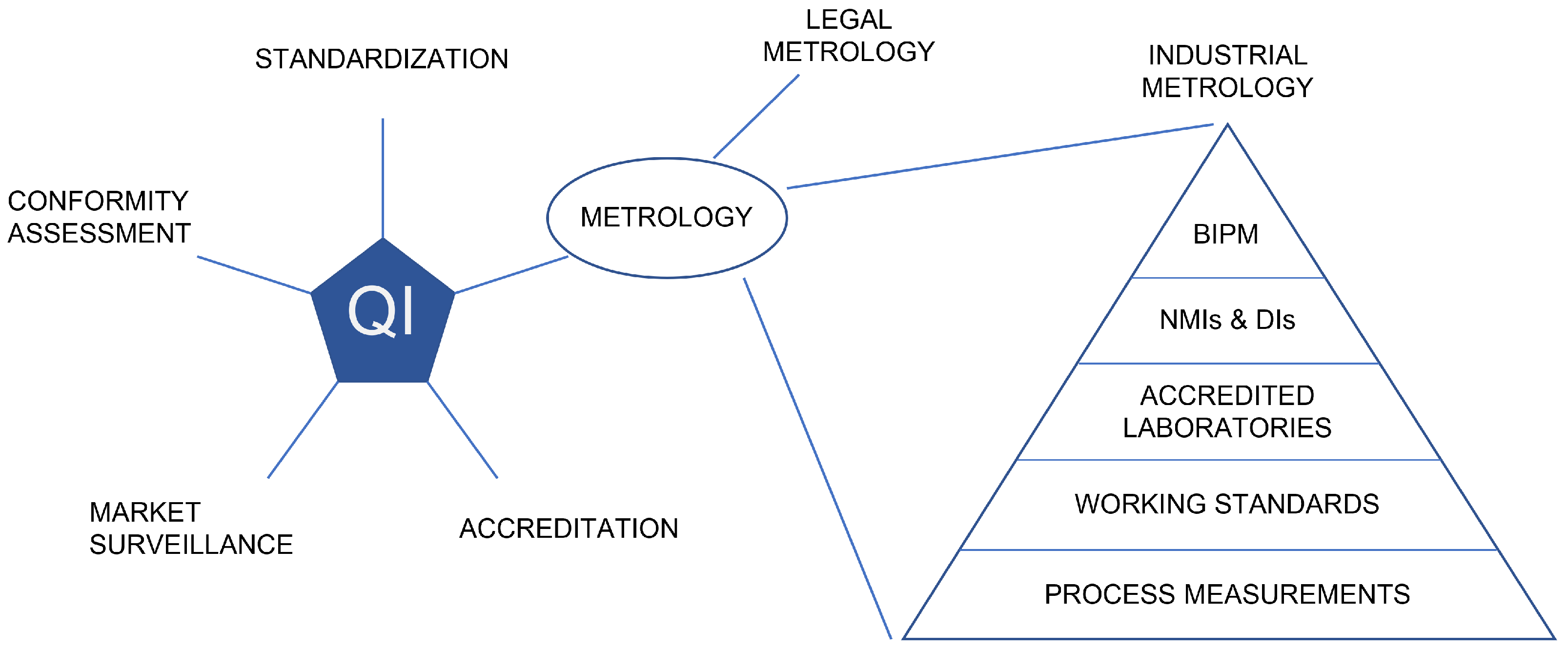
Whenever a product or service is brought to market, it is crucial that the product or service in question is safe for consumers and the environment. For these purposes, a global quality infrastructure (QI) consisting of both public and private organizations has been established. [1] The global cooperation of the QI is coordinated by the International Network on Quality Infrastructure (INetQI) [3], which defines QI as "the system comprising the organizations (public and private) together with the policies, relevant legal and regulatory framework, and practices needed to support and enhance the quality, safety and environmental soundness of goods, services and processes." [1] The cornerstones of QI are metrology, standardization, accreditation, conformity assessment, and market surveillance, which have their own sub-infrastructures, such as metrology infrastructure (MI), which is tied to its own international organizations. [1,2] Due to the differences in roles of the organizations and regulatory frameworks in the QI, there are organizations and regulatory bodies covering the different parts of the QI on national or regional levels. In turn, due to this division, there are possibilities for overlapping or conflicting regulations, which regulatory bodies ideally aim to avoid.
Currently, as significant efforts are being made to digitalize the QI [1,4,5,6,7], the differences in current practices and requirements quickly become apparent, causing challenges in the implementation of digital solutions. For ensuring that the data formats and digital processes meet their respective requirements and are applicable globally in different applications, harmonization is needed, as the current requirements may vary by the domain or region. Otherwise, it could be impossible, or at least highly cost-inefficient, for the organizations participating in the maintenance of the infrastructures, such as calibration laboratories and service providers, to adapt their services to be interoperable with the varying systems used by individual customers.
In this paper, we focus on the industrial part of the MI, in which the determining of the measurement uncertainty of individual instruments and traceability of measurements to the measurement unit system are established through calibrations. [8] As calibrations are a confirmative part of the quality management of processes that are based on measurements, they are not directly profitable operations but instead are necessary for avoiding prohibitive expenses and delays or fulfilling compliance requirements, allowing access to markets. For this reason, estimating the total economic benefits of the digital transformation of the MI for all the involved organizations individually is complex.
The same also applies to other similar processes in different parts of the QI. As the purpose of the QI, and thus also the MI, is mainly to oversee and support the industry in providing reliable and safe products and services to customers [1], most economical benefits from the digital transformation are formed in the customer end of the infrastructure. In the MI, this includes, e.g., manufacturing companies that can have several thousands of measurement instruments monitoring and controlling production lines and processes. For these companies, a significant part of the value of digitalization comes from the improvements in efficiency and reduced need for manual work through automation, which leads to savings from reduced human resources tied to quality management. In the case of calibrations, where the costs and possible savings from managing instrument data and information are relative to the number of the instruments that need to be regularly calibrated, these benefits will not be as significant for smaller service providers. This means that any investments in the digital transformation become difficult to justify without support and demand from the customers.
A good example of an industry that is reliant on MI is the pharmaceutical industry, where measurements provide the means for controlling the drug manufacturing processes. Thus, the quality and safety of pharmaceutical products are directly dependent on the reliability of process measurements. For this reason, ensuring the trustworthiness of the process instruments is an essential part of quality management in the pharmaceutical industry, which is why the measurement systems and their maintenance and calibration procedures are highly regulated. [9,10,11] Ensuring the data integrity of the measurements and any calibration-related data in particular is of the utmost importance for this purpose, which is why detailed records and audit trails for the processes are required. [12] Consequently, any processes including human operations, e.g., related to the documentation and handling of calibration data, will typically require several inspection and approval procedures to prevent the occurrence of human errors.
Since harmonization of data formats is essential for the interoperability and overall efficiency of digital systems, important areas requiring in-depth examination are the established standards, regulations, and guidelines that provide the framework for the current data formats and processes. Thus, a key question for the success of digitalization efforts becomes, "how compatible and applicable are the requirements defined for the different parties in QI in a fully digital environment?"
In general, the success of industry-wide transition in the digital environment relies on organizational capabilities to adapt to and uptake new technologies and systems when necessary. Thus, an important aspect of the digital transformation is ensuring the ease of adaptation, inexpensiveness, and sufficient scalability of the processes and systems so that the requirements of various types and sizes of organizations can be fulfilled. One possible solution for arranging these kinds of systems and services in a scalable manner is the utilization of a common cloud platform. An example of such a concept in the domain of metrology is the European Metrology Cloud project, aiming to advance the digitalization of legal metrology in Europe. [7,13]
Optimal digitalization of calibration data management requires a thorough understanding of the underlying processes and workflows. In this paper, we investigate the current practices and general requirements for calibration data management as a part of an overall quality management system (QMS) in the pharmaceutical industry. We analyze the significance and feasibility of the requirements for the harmonization of digital processes. The paper presents ways to optimize calibration data management processes, allowing improved efficiency by reduced manual work and removing the possibility of human errors in calibration data management. Thus, traceability and data integrity can be preserved between the organizations in the calibration chain. Furthermore, the proposed approach enables data analysis of the calibration data on a completely different scale compared to the printed A4/paper on glass (e.g., PDF) approach. A proof of concept (POC) of the method was developed by prioritizing the ease of adaptation to digital processes, and as such, the POC used systems already widely used in the industry, e.g., for user and access management. Thus, the set-up and operation of the platform required a less complex infrastructure compared to a fully proprietary infrastructure, as used by Thiel [13] and Bruns et al. [14]
To summarize, we present the following contributions beyond the state-of-the-art:
- Investigation of calibration procedures in the context of digital calibration certificates (DCC) [15], including the core standards and regulations affecting the pharmaceutical industry;
- Analysis of the applicability of the standards and regulations for the implementation of machine-proof data formats and application programming interfaces (APIs), acknowledging that the existing procedures, standards, and regulations have been prepared for human interpretation;
- Optimized digital data management processes for preserving traceability and data integrity in calibration chains;
- A concept for a multitenant platform for establishing ecosystems for collaborating organizations within the metrology infrastructure; and
- A proof of concept (POC) realization of points 3 and 4.
he paper is organized as follows. The next section provides the relevant background on the digitalization of metrology infrastructure, current developments in the use of internet of things (IoT) in manufacturing and quality management, and requirements for calibration data management in the process and pharmaceutical industries. Next, the harmonization of digitalized calibration data management based on the requirements for different organizations is discussed, followed by the proposed approach for the exchange of digital calibration data within the metrology infrastructure. The possibilities of the digital calibration data management, remaining challenges, and proposed research topics are discussed in the penultimate section, followed by conclusions.
Background
Metrology infrastructure as a part of the quality infrastructure
The worldwide MI is built upon standards, mutual trust, and recognition among organizations operating around the world. [16] To make the infrastructure as comprehensive as possible, it consists of organizations with different hierarchical roles. At the top of the hierarchy are the national metrology institutes (NMIs) and designated institutes (DI), which maintain the SI system and metrological standards jointly defined by the International Bureau of Weights and Measures (BIPM). Figure 1 illustrates how the MI is established as a part of the QI. The hierarchy of the MI is often depicted in the form of a triangle or pyramid as the amount of measurement instruments and references increases when moving from the NMIs towards the industrial measurement application. (A few examples of how the MI has been implemented nationally in European countries are given in the work of Nikander et al.[17].)
|
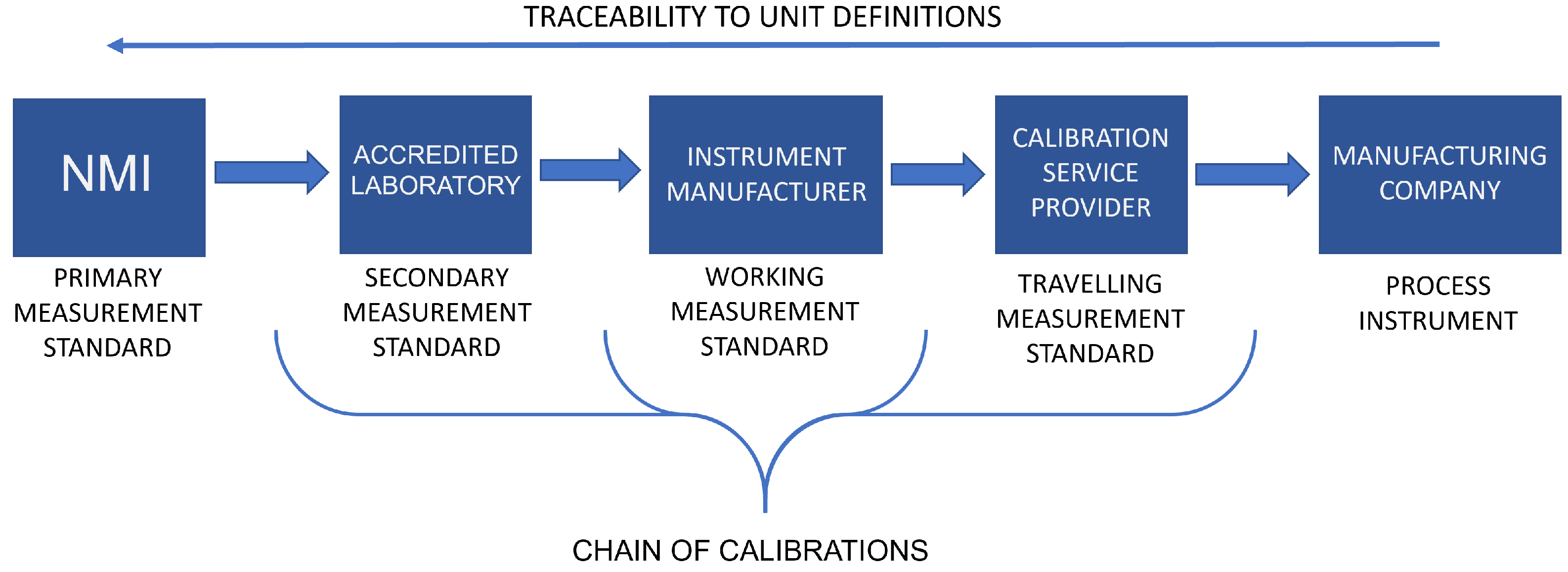
The measurement standards maintained by the NMIs or DIs are referred to as primary standards, as their purpose is to provide as accurate and precise physical representations of the unit definitions as possible. Between the industrial applications and primary standards are secondary measurement standards, which are maintained by accredited laboratories and calibrated by the NMIs or DIs. Calibrations are essential to maintain the traceability to SI unit definitions, which ensures the comparability and trustworthiness of measurement results given by individual instruments. [8] Depending on the applications, additional levels of references may also be used in the industry. For example, in the pharmaceutical industry, where the number of instruments used for monitoring and controlling the manufacturing processes can be several thousand per manufacturing site, the use of working standards and travelling standards is common as they provide efficiency in terms of time and costs, e.g., by allowing the calibrations of the process instruments to be carried out on-site. Figure 2 shows an example of a calibration chain from the primary standard of an NMI, all the way through to a process instrument of a pharmaceutical manufacturing company.
|
Due to the complexity of the MI and diversity of the organizations involved, the progress of digitalization has been slow, and in many cases data are still being managed in paper or paper-on-glass formats, requiring human interpretation, e.g., in the case of documenting calibrations in calibration certificates. [15] For field calibrations, digitalized solutions have become more common, as the costs of calibrations are proportional to the efficiency of the processes. However, these solutions tend to be instrument- and system-provider-specific, and due to competition, the willingness of the system providers to collaborate by harmonizing their systems and making them more open has been low. In addition, it is common that larger industrial companies cooperate with several instrument manufacturers and service providers. Consequently, in the worst case for the instrument owner, the data exchange from different organizations has been scattered in several systems using different formats. Because of this, human resources relative to the number of annual calibrations have been tied up in calibration management alone to ensure compliance with quality management regulations, meaning that it has been a significant expense.
As digitalization in the industry and other sectors is progressing, and the collection and use of data is growing rapidly, the topics concerning data quality and trustworthiness become increasingly important, which is driving the research and development efforts in digitalization within the metrology community. [7,18] The ongoing efforts include the definition and development of digital formats for presenting metrological information, e.g., data models for presenting metrologically relevant data as semantic metadata [19,20,21], or formats for digital calibration certificates. [15,22,23,24] For this work, the exchange of DCCs was tested using the DCC format originally defined by the Physikalisch-Technische Bundesanstalt (PTB) and further developed in EMPIR SmartCom and Gemimeg research projects. [4,5,25,26,27,28]
Digitization of the data formats sets new requirements for the development of the infrastructure, as its operation has been based on mutual recognition and trust between organizations. [16] Transition to a digital environment also means that this trust needs to be established digitally to enable its representation in a machine-understandable format. [29,30,31] For this purpose, data security and cryptographical solutions, e.g., the use of digital signatures, are relevant. [15,17]
IoT in pharmaceutical and process industries
The digital transformation of the manufacturing industry is typically referred to as Industry 4.0, based on the significance and potential of digitalization in industrial settings. The key technologies of Industry 4.0 include smart IoT devices and sensors that enable the collection of vast amounts of data from different manufacturing processes and operations. Combined with the technological advancements in computing power, analyzing methods for big data and machine learning, Industry 4.0 enables significant improvements in the efficiency of manufacturing processes. [32]
In addition to automation, digitalization also provides new possibilities to enhance the work where automation is not feasible or needs to be supported by manual operations, i.e., smart working. Smart-working-enabling technologies include, e.g., wearable IoT devices and augmented reality, which aid the interaction between the operators and manufacturing systems. [33] IoT technologies can also be used to improve worker safety in the working environment. [34]
One of the main technologies that is being studied and developed based on Industry-4.0-enabling technologies is cyber-physical systems, i.e., digital twins, in which, e.g., data, simulation models, and predictive analytics are used to form as accurate a digital representation of a physical entity as possible to further improve the possibilities to analyze their performance and behavior. [35,36,37,38]
In terms of quality management, the effects of Industry 4.0 are prominent in the digitalization of methodologies such as total quality management (TQM) and the use of IoT technologies in quality management operations and processes. [39] In some cases, the advancements in IoT technologies have also led to situations where traditional quality management is not in alignment with the requirements of IoT devices. [40]
In the process and pharmaceutical industries specifically, the benefits of IoT and Industry 4.0 can be seen as being the same as in other manufacturing industries. [41,42] However, in the pharmaceutical industry, digitalization has been slowed due to the interdependence of processes and strict regulations requiring, e.g., comprehensive validation of newly developed systems and software, due to which the investments in Industry 4.0 solutions can be both risky and expensive. [43,44,45] In a survey by Reinhardt et al., the main areas of focus for Industry 4.0 adoption in the pharmaceutical industry were optimizing processes, monitoring production performance, maintaining regulatory compliance, and minimizing production downtime. [46]
Requirements for the calibration data management and data formats
Section 7.6 of the ISO 9001 standard for QMSs contains the general requirements for the handling of measuring and monitoring equipment. [47] The standard requires that the equipment used for quantitative measurements must be calibrated periodically, for which the instrument owner must define and document an operational procedure.
For the process and pharmaceutical industry, there are legislative requirements for the federal legislations, e.g., pharmacopeias and good practices (GxP) such as good manufacturing practice (GMP) and good laboratory practices (GLP).
The main standard defining the requirements for calibration data management and certificate formats for accredited laboratories is the ISO/IEC 17025 standard on general requirements for the competence of testing and calibration laboratories. [48] This standard, accompanied by other more general standards such as ISO 9001, is used as the basis for the accreditation of calibration laboratories. [49] The requirements for the contents of a calibration certificate are defined in Section 7.8 of the ISO/IEC 17025 standard.
General guidelines and good practices
In addition to international standards and regional legislations, there also exist several non-obligatory recommendations in the form of good practices or guidelines by different organizations, which have become widely used and unofficially agreed de facto standards for the industry. These de facto standards can be guidelines by NMIs or regional metrology organizations (RMO) on calibration procedures for specific instrument types, guidelines by societies and associations of the experts in the field [50,51], or guides and whitepapers by companies, e.g., instrument manufacturers, which are typically based on the relevant legislative requirements.
Mass as an example measurand
The differences that the standards, regulations, and guidelines of the different organizations may have can be pointed out by examining a singular physical quantity and the respective measurement methodologies. Harmonization is beneficial, especially given that differences in the calibration procedures can result in differences, e.g., in uncertainty evaluations. [52] Good examples of the harmonization of practices on a global level are the most recent revisions of the regulations and guidelines for mass and weighing, e.g., non-automatic weighing instruments.
Standards covering the use of non-automatic weighing instruments include the European EN 45501:2015 Metrological aspects of non-automatic weighing instruments [53] and the American NIST Handbook 44 Specifications, Tolerances, and Other Technical Requirements for Weighing and Measuring Devices. [54]
Regional guidelines for the calibration procedures for weighing instruments are published by the RMOs. In the case of non-automatic weighing instruments, these guidelines include, for example, Euramet Calibration Guideline No. 18 Guidelines on the Calibration of Non-Automatic Weighing Instruments [55], ASTM E898 Standard practice for calibration of non-automatic weighing instruments [56], SIM MWG7 Guidelines on the calibration of non-automatic weighing instruments [57], and JJF 1847 Calibration Specifications for Electronic Balances. [58] Harmonization of these guidelines has been based on the other organizations adapting their guidelines to be commensurate with the EURAMET CG-18, which has also become better known in the Asian region, allowing the requirements to become a global de facto standard. [11]
At the industrial level, the requirements for the weighing instruments and other measurement instruments are defined in the regional or national regulations for pharmaceutical products. In the US Pharmacopeia (USP), the requirements for balances are covered in General Chapter 41 “Balances,” [9] with supporting guidance given in General Chapter 1251 “Weighing on an Analytical Balance.” [59] Respectively, in the European Pharmacopoeia, the requirements for balances are covered in General Chapter 2.1.7 “Balances for analytical purposes.” [10] After the latest revision of the European Pharmacopoeia, the requirements are commensurate with the USP, which has been a welcome advancement towards the harmonization of regulations. [11]
Requirements for data integrity
One of the topics where improvements are pursued by investing in digitalization in the pharmaceutical industry is data integrity. The basis for data integrity regulation compliance is typically referred to as the ALCOA+ principle [60,61], the definition of which is presented in Table 1.
| ||||||||||||||||||||||||
Regulatory requirements for data integrity in manufacturing are either defined as a part of the GMPs and GLPs or in separate regulations such as the US Food and Drug Administration's (FDA's) Title 21 Code of Federal Regulations Part 11 (21 CFR Part 11) [62] or the Medicines & Healthcare Products Regulatory Agency's (MHRA's) Data Integrity Guidance and Definitions. [63] An example of a de facto standard on data integrity is the ISPE GAMP Guide: Records & Data Integrity [12], where data integrity requirements defined in the regulations are approached in a more practical way.
Data integrity is also covered, although to a lesser extent, in Section 7.11 of ISO/IEC 17025, where requirements for data management systems used by calibration laboratories for collecting, processing, recording, reporting, storing, or retrieving data are defined. [48] However, as the requirements for the calibration certificates focus on the content that must be included, regardless of whether the certificate is a physical or electronic document, the standard does not specify detailed requirements or methods for securing a digital file.
Harmonization challenges for developing digital solutions
The challenge in developing harmonized and interoperable formats and data exchange between the calibration service providers and the customers is that the requirements for the process and its documentation depend on the roles of the organizations in a calibration chain. For the NMI's and accredited laboratories, the calibration practices are mostly defined in the ISO/IEC 17025 standard and other requirements that are mandated as a part of the accreditation.
However, a significant amount of the calibrations performed in the industry are not done according to the ISO/IEC 17025 standard, mostly due to costs. This includes field calibrations that are performed on-site, as this is far more cost-efficient in comparison to having all the process-controlling instruments calibrated at a separate laboratory. For these calibrations, the requirements are defined in the quality management regulations and legislation of the national and regional authorities. Compared to ISO/IEC 17025, which specifically focuses on calibrations, legislative requirements focus on the QMS as a whole. The main principles regarding the calibrations of the instruments are no different, but the context and reasoning behind them is.
For NMIs and accredited laboratories, calibrations are a service that they provide for their customers, and they are regulated and audited to ensure that their processes are up to the level that is required; this is why the requirements are very specific. On the other hand, in the industry’s end of the calibration chain, the regulations are not as specifically defined in all cases. For example, instead of requiring, e.g., calibration processes to follow a specifically defined procedure, companies are mandated to have written instructions for the processes, meaning that some of the details are left open for companies to define themselves. Due to this openness, there are some guidelines and good practices that have been adopted as de facto standards for calibration procedures. To shortly summarize the differences between the regulations for accredited calibrations and field calibrations, accredited calibrations are focused on an individual instrument, whereas field calibrations consider the instrument a part of a process line.
The effects of these differences in the requirements can be noticed when comparing a typical format for a field calibration certificate to a certificate issued by an accredited laboratory. The field calibration certificates must include information such as the identification of the position or location where the instrument has been used in a particular process line—which is relevant information for the quality assurance of that process line—and a calibration due date based on the calibration interval specified in the manufacturing company’s requirements.
For the calibration data, the main difference is that the calibration data have been presented in a different way. The standards for the accredited laboratories require that the calibration results are presented, including the measurement uncertainties of the measurement points, whereas in the regulations for the industry, it is relatively common that the deviations or measurement errors are required. Another example of these kinds of differences includes requirements for the use of different units in different parts of the world.
In addition to the variances in requirements, some challenges for the digitalization of the processes arise from the way in which the requirements have been defined in the standards and legislation, as the current formats are written for human interpretation. The International Electrotechnical Commission (IEC) has presented a utility model for SMART Standards. [64] The model defines the following levels for the digitization degree of a standard:
- Level 0: Paper format - This format is not suitable for direct automatic processing or usage.
- Level 1: Digital format (e.g., PDF) - This format allows automatic management and display of the document.
- Level 2: Machine-readable format - The structure of the document can be digitized, and certain granular content can be exported (chapters, graphics, definitions, etc.). Content and presentation are separated.
- Level 3: Machine-readable and -executable content - All essential granular information units can be clearly identified, and their reciprocal relationships can be recorded and made available for further processing or partial implementation.
- Level 4: Machine-interpretable content - The information in a standard is linked with implementation and usage information in such a way that it is implemented by machines directly or interpreted and combined with other information sources so that complex actions and decision-making processes take place automatically.
The German Institute for Standardisation (DIN) and German Association for Electrical, Electronic & Information Technologies (DKE) have presented an additional level that goes beyond Level 4 in the IEC model [64]:
- Level 5: Machine-controllable content - The content of a standard can be amended by machines working unassisted and adopted by automated (distributed) decision-making processes. The content adopted in this way is automatically reviewed and published via the publication channels of the standardization organizations.
The model aptly emphasizes the difference among machine-readable, -executable, -interpretable, and -controllable formats. Ideally, for the development of digital calibration management processes, the requirements would have to be machine-interpretable, enabling requirement-specific data and criteria such as allowance limits to be directly interpreted by the management systems as process parameters, making further automation of the processes management possible. Currently, when the requirements are not yet available in such a format, this kind of specific information must be included in the systems through human interpretation, which means that revisions of the requirements will require updating of the systems accordingly.
Materials and methods
Studied cases for the proof of concept
In the proof of concept (POC), the focus of the work was to harmonize the data exchange between the parties of a calibration chain to enable automation of the data management processes in the receiving end, thus eliminating the need for manual work to interpret and input the data into calibration management systems (CMS) or enterprise resource planning (ERP) systems, making the processes more efficient and improving data integrity by reducing the chance of input errors. To achieve these goals, use of a multitenant platform was chosen as the basis for defining the data exchange processes and methods.
The DCC formats used in the POCs were the DCC XML schema versions 2.4.0[1] in Case 1 and 3.0.0-rc2[2] in Case 2. In both cases, the means of using the DCC schema were agreed upon based on the requirements and needs of the participating organizations representing the calibration customer. The DCCs used in the testing were developed based on examples of existing calibration certificates.
Case 1
In Case 1, the exchange and handling of DCCs was tested by mimicking a realistic calibration chain from a national measurement standard of an NMI to a working standard of a pharmaceutical manufacturing company. The main objectives for the POC were achieving an efficient and easy-to-use method for managing calibrations and the related data exchange between organizations. The physical quantities for which the harmonized file formats were agreed upon within the consortium were temperature and pressure, as the working standard in question is capable of measuring both quantities. In addition to Aalto University, the organizations involved were:
- VTT MIKES, the Finnish NMI;
- Vaisala Oyj, an instrument manufacturer and a provider of calibration services, with an accredited laboratory;
- Orion Oyj, a pharmaceutical company; and
- Beamex Oy Ab, a field calibration instrument and CMS provider.
Figure 3 shows the POC setting, where different colors highlight the systems and operations of the organizations involved.
|
References
- ↑ Physikalisch-Technische Bundesanstalt (2019). "xmlns:dcc schema version 2.4.0". https://www.ptb.de/dcc/v2.4.0/dcc.xsd. Retrieved 06 June 2022.
- ↑ Physikalisch-Technische Bundesanstalt (2019). "xmlns:dcc schema version 3.0.0-rc.2". https://www.ptb.de/dcc/v3.0.0-rc.2/dcc.xsd. Retrieved 06 June 2022.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Grammar was tweaked significantly to improve readability. The PMCID and DOI were also added when they were missing from the original reference. The links to the DCC XML schemas of the original article were turned into proper references for this version.