Difference between revisions of "Journal:Practical considerations for laboratories: Implementing a holistic quality management system"
Shawndouglas (talk | contribs) (Created stub. Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 6: | Line 6: | ||
|title_full = Practical considerations for laboratories: Implementing a holistic quality management system | |title_full = Practical considerations for laboratories: Implementing a holistic quality management system | ||
|journal = ''Frontiers in Bioengineering and Biotechnology'' | |journal = ''Frontiers in Bioengineering and Biotechnology'' | ||
|authors = Pillai, | |authors = Pillai, Segaran; Calvery, Jennifer; Fox, Elizabeth | ||
|affiliations = Food and Drug Administration, Booz Allen Hamilton | |affiliations = Food and Drug Administration, Booz Allen Hamilton | ||
|contact = Email: Segaran dot Pillai at fda dot hhs dot gov | |contact = Email: Segaran dot Pillai at fda dot hhs dot gov | ||
| Line 26: | Line 26: | ||
}} | }} | ||
==Abstract== | ==Abstract== | ||
A [[quality management system]] (QMS) is an essential element for the effective operation of research, clinical, testing, or production/manufacturing [[Laboratory|laboratories]]. As technology continues to rapidly advance and new challenges arise, laboratories worldwide have responded with innovation and process changes to meet the continued demand. It is critical for laboratories to maintain a robust QMS that accommodates laboratory activities (e.g., basic and applied research; regulatory, clinical, or proficiency testing), records management, and a path for [[Continual improvement process|continuous improvement]] to ensure that results and data are reliable, accurate, timely, and reproducible. A robust, suitable QMS provides a framework to address gaps and risks throughout the laboratory's [[workflow]] that could potentially lead to a critical error, thus compromising the integrity and credibility of the institution. While there are many QMS frameworks (e.g., a model such as a consensus standard, guideline, or regulation) that may apply to laboratories, ensuring that the appropriate framework is adopted based on the type of work performed and that key implementation steps are taken is important for the long-term success of the QMS and for the advancement of science. Ultimately, a well-considered QMS ensures accurate results, efficient operations, and increased credibility, enabling protection of [[public health]] and safety. Herein, we explore QMS framework options for each identified laboratory category and discuss prerequisite considerations for implementation. An analysis of various frameworks’ principles and conformity requirements demonstrates the extent to which they address basic components of effective laboratory operations and guides optimal implementation to yield a holistic, sustainable framework that addresses the laboratory’s needs and the type of work being performed. | A [[quality management system]] (QMS) is an essential element for the effective operation of [[research]], clinical, testing, or production/manufacturing [[Laboratory|laboratories]]. As technology continues to rapidly advance and new challenges arise, laboratories worldwide have responded with innovation and process changes to meet the continued demand. It is critical for laboratories to maintain a robust QMS that accommodates laboratory activities (e.g., basic and applied research; regulatory, clinical, or proficiency testing), records management, and a path for [[Continual improvement process|continuous improvement]] to ensure that results and data are reliable, accurate, timely, and reproducible. A robust, suitable QMS provides a framework to address gaps and risks throughout the laboratory's [[workflow]] that could potentially lead to a critical error, thus compromising the integrity and credibility of the institution. While there are many QMS frameworks (e.g., a model such as a consensus standard, guideline, or regulation) that may apply to laboratories, ensuring that the appropriate framework is adopted based on the type of work performed and that key implementation steps are taken is important for the long-term success of the QMS and for the advancement of science. Ultimately, a well-considered QMS ensures accurate results, efficient operations, and increased credibility, enabling protection of [[public health]] and safety. Herein, we explore QMS framework options for each identified laboratory category and discuss prerequisite considerations for implementation. An analysis of various frameworks’ principles and conformity requirements demonstrates the extent to which they address basic components of effective laboratory operations and guides optimal implementation to yield a holistic, sustainable framework that addresses the laboratory’s needs and the type of work being performed. | ||
'''Keywords''': quality, quality management system, laboratory, implementation, framework, total quality, reproducibility | '''Keywords''': quality, quality management system, laboratory, implementation, framework, total quality, reproducibility | ||
==Introduction== | ==Introduction== | ||
In order for [[Laboratory|laboratories]] to support the [[public health]] mission and better address emerging public health challenges, the need for a [[quality management system]] (QMS) that facilitates risk-based thinking and enhances assurance of [[data quality]] becomes more critical. With the worldwide shock of the [[coronavirus disease 2019]] (COVID-19) outbreak, organizations have been forced to pivot and innovate to address new issues. The rapid response across industries towards COVID-19 and other crises illustrates business resilience and emphasizes the importance of [[Quality (business)|quality]] management in the laboratory and its organizational culture. However, incidents stemming from gaps in quality management and affecting public health continue to occur. Laboratory errors have a reported frequency of 0.012%–0.6% of all test results, which in turn has huge impact on diagnosis, as 60 to 70% of all diagnoses are made based upon laboratory tests. [Agarwal, 2014] In addition, there is evidence of an observed data reproducibility crisis in the research community that would benefit from attention and improvement. A recent ''Nature'' survey of 1,576 researchers found that 52% of those surveyed agree that there is a crisis of reproducibility; the same survey found that over 70% have failed to reproduce another scientist’s data. [Baker, 2016] | In order for [[Laboratory|laboratories]] to support the [[public health]] mission and better address emerging public health challenges, the need for a [[quality management system]] (QMS) that facilitates risk-based thinking and enhances assurance of [[data quality]] becomes more critical. With the worldwide shock of the [[coronavirus disease 2019]] (COVID-19) outbreak, organizations have been forced to pivot and innovate to address new issues. The rapid response across industries towards COVID-19 and other crises illustrates business resilience and emphasizes the importance of [[Quality (business)|quality]] management in the laboratory and its organizational culture. However, incidents stemming from gaps in quality management and affecting public health continue to occur. Laboratory errors have a reported frequency of 0.012%–0.6% of all test results, which in turn has huge impact on diagnosis, as 60 to 70% of all diagnoses are made based upon laboratory tests. [Agarwal, 2014] In addition, there is evidence of an observed data reproducibility crisis in the [[research]] community that would benefit from attention and improvement. A recent ''Nature'' survey of 1,576 researchers found that 52% of those surveyed agree that there is a crisis of reproducibility; the same survey found that over 70% have failed to reproduce another scientist’s data. [Baker, 2016] | ||
A QMS has numerous benefits that contribute to managing risks in the laboratory, including errors, and mitigating reproducibility crisis concerns. One major benefit is enabling the laboratory to better identify, assess, and address risks faced in the laboratory at all levels of the organization. Poor data quality is one such risk. Some standards, such as the International Organization for Standardization's (ISO's) ISO 31000, are completely dedicated to [[risk management]] [International Organization for Standardization, 2018], while other more holistic quality management standards, guidelines, and regulations—such as [[ISO/IEC 17025]]—incorporate the concept of risk-based thinking throughout their respective frameworks. [International Organization for Standardization, 2017] In turn, risk management and other aspects of quality management can be built into all three phases of the workflow path (commonly referred to as the "total testing process") of a laboratory: pre-analytical, analytical, and post-analytical. [World Health Organization, 2011] | A QMS has numerous benefits that contribute to managing risks in the laboratory, including errors, and mitigating reproducibility crisis concerns. One major benefit is enabling the laboratory to better identify, assess, and address risks faced in the laboratory at all levels of the organization. Poor data quality is one such risk. Some standards, such as the International Organization for Standardization's (ISO's) ISO 31000, are completely dedicated to [[risk management]] [International Organization for Standardization, 2018], while other more holistic quality management standards, guidelines, and regulations—such as [[ISO/IEC 17025]]—incorporate the concept of risk-based thinking throughout their respective frameworks. [International Organization for Standardization, 2017] In turn, risk management and other aspects of quality management can be built into all three phases of the workflow path (commonly referred to as the "total testing process") of a laboratory: pre-analytical, analytical, and post-analytical. [World Health Organization, 2011] | ||
| Line 37: | Line 37: | ||
The pre-analytic phase of a laboratory’s workflow encompasses the activities that are completed prior to operational testing and research (e.g., [[Sample (material)|sample]] collection and transport). These activities are performed to prepare and support the laboratory in its operations, research, and services. The analytic phase of a laboratory’s workflow encompasses the activities that are completed within the laboratory during operational testing and research. These activities are performed to directly execute the laboratory’s operations, research, and services (e.g., sample testing, experimental studies). Finally, the post-analytic phase of a laboratory’s workflow encompasses the activities that are completed after operational testing and research (e.g., reporting). These activities assure that processes are conducted in a manner that ensures compliance, accuracy, customer satisfaction, and continual improvement. | The pre-analytic phase of a laboratory’s workflow encompasses the activities that are completed prior to operational testing and research (e.g., [[Sample (material)|sample]] collection and transport). These activities are performed to prepare and support the laboratory in its operations, research, and services. The analytic phase of a laboratory’s workflow encompasses the activities that are completed within the laboratory during operational testing and research. These activities are performed to directly execute the laboratory’s operations, research, and services (e.g., sample testing, experimental studies). Finally, the post-analytic phase of a laboratory’s workflow encompasses the activities that are completed after operational testing and research (e.g., reporting). These activities assure that processes are conducted in a manner that ensures compliance, accuracy, customer satisfaction, and continual improvement. | ||
Each of these three phases must be addressed when implementing a QMS. While someone may suppose that the highest risk of error would occur in the analytic phase, there is now incontrovertible evidence that the majority of laboratory errors occur in the pre-analytical phase (61.9%–68.2%), which are followed by mistakes in the post-analytical phase (18.5%–23.1%) and analytical phase (13.3%–15%). [Mrazek et al., 2020] Within the path of workflow, laboratory processes and procedures can be organized into 12 management areas, known as quality system essentials (QSEs); the 12 QSEs are globally recognized principles that address all aspects of a QMS and cover the entire path of workflow of a laboratory. When all of the laboratory procedures and processes are organized into an understandable and workable structure, the opportunity to ensure that all are appropriately managed is increased. [World Health Organization, 2011] Moreover, as safety is a key QSE | Each of these three phases must be addressed when implementing a QMS. While someone may suppose that the highest risk of error would occur in the analytic phase, there is now incontrovertible evidence that the majority of laboratory errors occur in the pre-analytical phase (61.9%–68.2%), which are followed by mistakes in the post-analytical phase (18.5%–23.1%) and analytical phase (13.3%–15%). [Mrazek et al., 2020] Within the path of workflow, laboratory processes and procedures can be organized into 12 management areas, known as quality system essentials (QSEs); the 12 QSEs are globally recognized principles that address all aspects of a QMS and cover the entire path of workflow of a laboratory. [CLSI clsi.org/standards-development/quality-system-essentials/] When all of the laboratory procedures and processes are organized into an understandable and workable structure, the opportunity to ensure that all are appropriately managed is increased. [World Health Organization, 2011] Moreover, as safety is a key QSE [CLSI clsi.org/standards-development/quality-system-essentials/], laboratories such as those handling pathogens and other biosafety risks have the opportunity to evaluate the effectiveness of their biosafety procedures and implement improvements (e.g., take measures to reduce the risk of contamination). An integrated, robust QMS can help the laboratory cope with uncertainties that naturally occur in all laboratory environments. | ||
In his 1993 book ''Preventing Chaos in a Crisis: Strategies for Prevention, Control, and Damage Limitation'', Patrick Lagadec emphasizes that the response to an emergency cannot be developed unless the institution has prepared to adapt [Lagadec, 1993]: | In his 1993 book ''Preventing Chaos in a Crisis: Strategies for Prevention, Control, and Damage Limitation'', Patrick Lagadec emphasizes that the response to an emergency cannot be developed unless the institution has prepared to adapt [Lagadec, 1993]: | ||
| Line 44: | Line 44: | ||
Given the naturally complex operations of the modern laboratory, minimizing preventable problems and applying lessons learned should be a primary goal. | Given the naturally complex operations of the modern laboratory, minimizing preventable problems and applying lessons learned should be a primary goal. | ||
==Defining major laboratory categories== | |||
Laboratories are distributed widely across the United States and deal with diverse and unique challenges as a result of differing locations, type of work being performed, and federal, state and local regulations. The role of modern laboratories is quickly evolving as the delivery of health care undergoes drastic changes in the face of unprecedented challenges. With drastic changes comes the need to effectively manage them to ensure continued quality of products and services. It will be critical for a laboratory, belonging to any of the below categories, to reevaluate its alignment with the organization’s strategic direction and quality policy, continually [[Risk assessment|assess risk]] and impact of changes, carefully define organizational roles and responsibilities, and foster a strong culture of quality and safety. | |||
===Testing laboratories (including regulatory laboratories)=== | |||
Testing laboratories conduct conformance testing to ensure the safety, efficacy, and security of a vast array of materials, including, but not limited to, human and veterinary drugs, biological products, [[medical device]]s, food, water, cosmetics, radiation-emitting products, and tobacco products. Testing laboratories are driven by and generate results to support [[Regulatory compliance|statutory obligations]] to protect the public and minimize risk associated with such products or materials. | |||
===Product development and manufacturing laboratories=== | |||
Product development and manufacturing laboratories are a subset of testing laboratories that conduct routine [[quality control]] analysis, as well as pre-market research and development analysis during design phases, in order to ensure conformity of manufactured products (e.g., test reagents, devices to support analysis, testing or diagnostics) against a set of criteria or attributes selected and agreed upon by the institution. Product development and manufacturing laboratories are driven largely by regulatory requirements, or customer requirements and satisfaction. | |||
===Basic and applied research laboratories=== | |||
Research laboratories can be divided into basic and applied research units. While there is some natural overlap between the two, there are key differences. Basic research is conducted to seek understanding and form answers to scientific questions. According to the Association of American Medical Colleges (AAMC), basic science research—often called fundamental or bench research—provides the foundation of knowledge for the applied science that follows. [AAMC www.aamc.org/what-we-do/mission-areas/medical-research/basic-science] Applied research laboratories conduct studies that are designed to solve practical problems. While considered separate entities from regulatory laboratories, basic and applied research laboratories conduct their work to support and inform the understanding of science, (such as pathogenesis, side effects, efficacy of medical countermeasures, development and evaluation of technology and devices, etc.) and to support regulatory requirements. | |||
===Proficiency testing laboratories=== | |||
Proficiency testing laboratories assess the performance of individual laboratories responsible for specific tests or measurements generating data for regulatory consideration. Results are used to evaluate laboratories’ continuing performance, an important aspect of laboratory quality management and ensuring [[data integrity]]. | |||
===Clinical laboratories=== | |||
[[Clinical laboratory|Clinical laboratories]] conduct a variety of testing on clinical specimens to evaluate patients’ health. Their work aids in the diagnosis, treatment, and prevention of disease. The [[Centers for Medicare and Medicaid Services]] (CMS) regulates clinical laboratory testing performed on human specimens in the United States through the [[Clinical Laboratory Improvement Amendments]] (CLIA). [Center for Medicare and Medicaid Services, 2020] | |||
==Recommendation: Identify the correct laboratory quality management system framework for the type of laboratory work conducted== | |||
Laboratories should strive to gain a thorough understanding of their current state, goals, and objectives prior to selecting and implementing a QMS framework. Determining the major category of the laboratory will help to narrow the focus of the target state and identify if there is any overlapping of categorization that can be addressed in different ways. Capturing the current needs of the laboratory, customers/stakeholders and their requirements, and applicable rules and regulations for which compliance is required will help the laboratory to understand the current state in order to identify the most suitable standard or framework that will govern their QMS and serve as the benchmark for the target state. Once the laboratory's requirements are understood, an appropriate framework or standard for the QMS can then be selected. The subsequent sections provide an analysis of well-known QMS frameworks that can serve to guide laboratories in selecting the most appropriate model for implementation, beginning with a framework crosswalk analysis. | |||
===Crosswalk of the quality system essentials and quality management standard requirements=== | |||
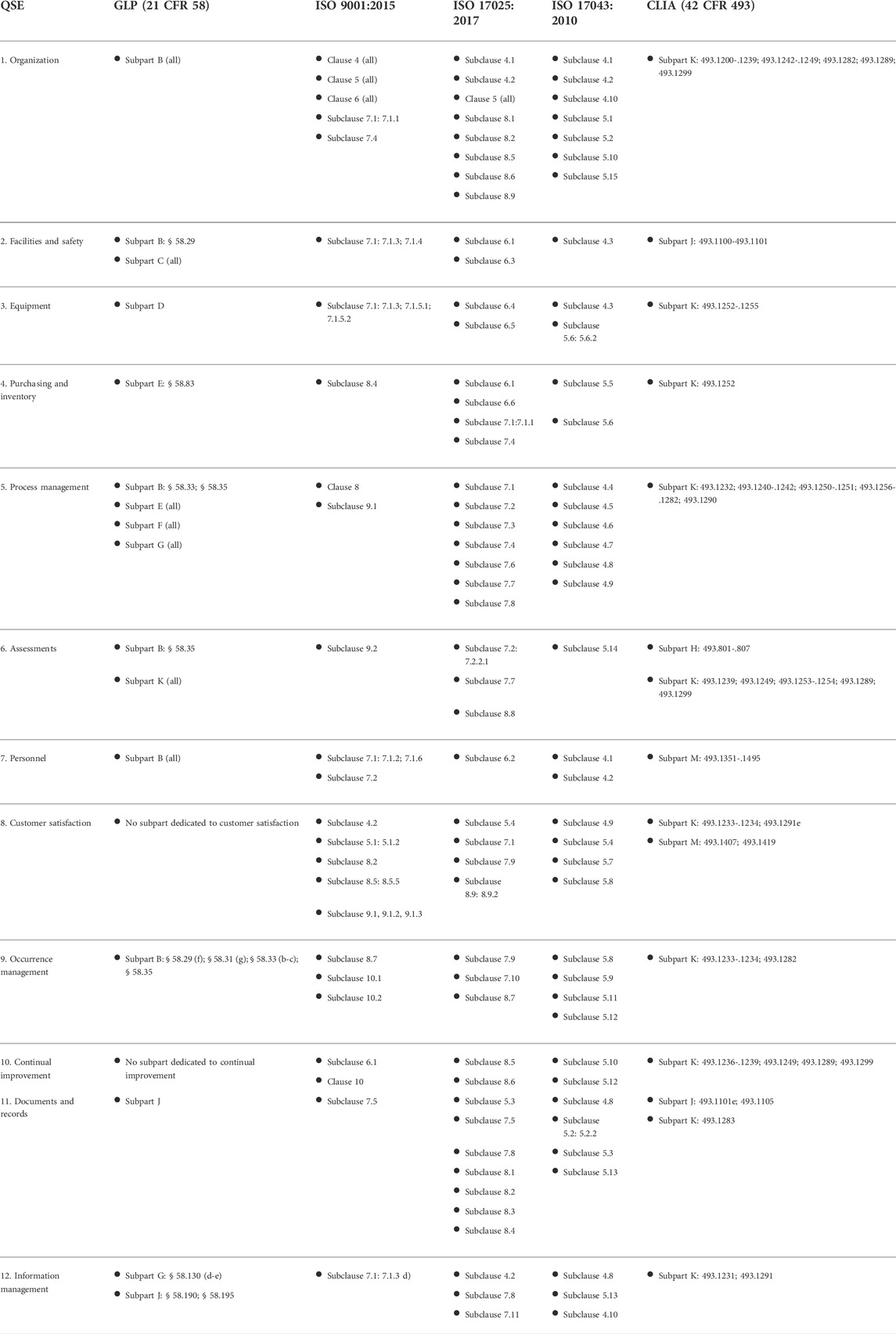
Table 1 presents a crosswalk analysis of well-known quality management framework documents against the framework of 12 QSEs. These QSEs cover all basic elements of a laboratory's QMS. Each standard was reviewed against the QSE framework, aligning a section (e.g., clause, subclause, subpart) to the applicable QSE in order to estimate the coverage of laboratory quality for the given quality management standard. | |||
[[File:Fig1 Pillai FrontBioengBiotech2022 10.jpg|1145px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="1145px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Table 1.''' Crosswalk of common quality management framework documents. Note that this table displays a non-exhaustive list of example applicable clauses, subclauses, subparts, and requirements.</blockquote> | |||
|- | |||
|} | |||
|} | |||
===Analysis of quality management standards by laboratory category=== | |||
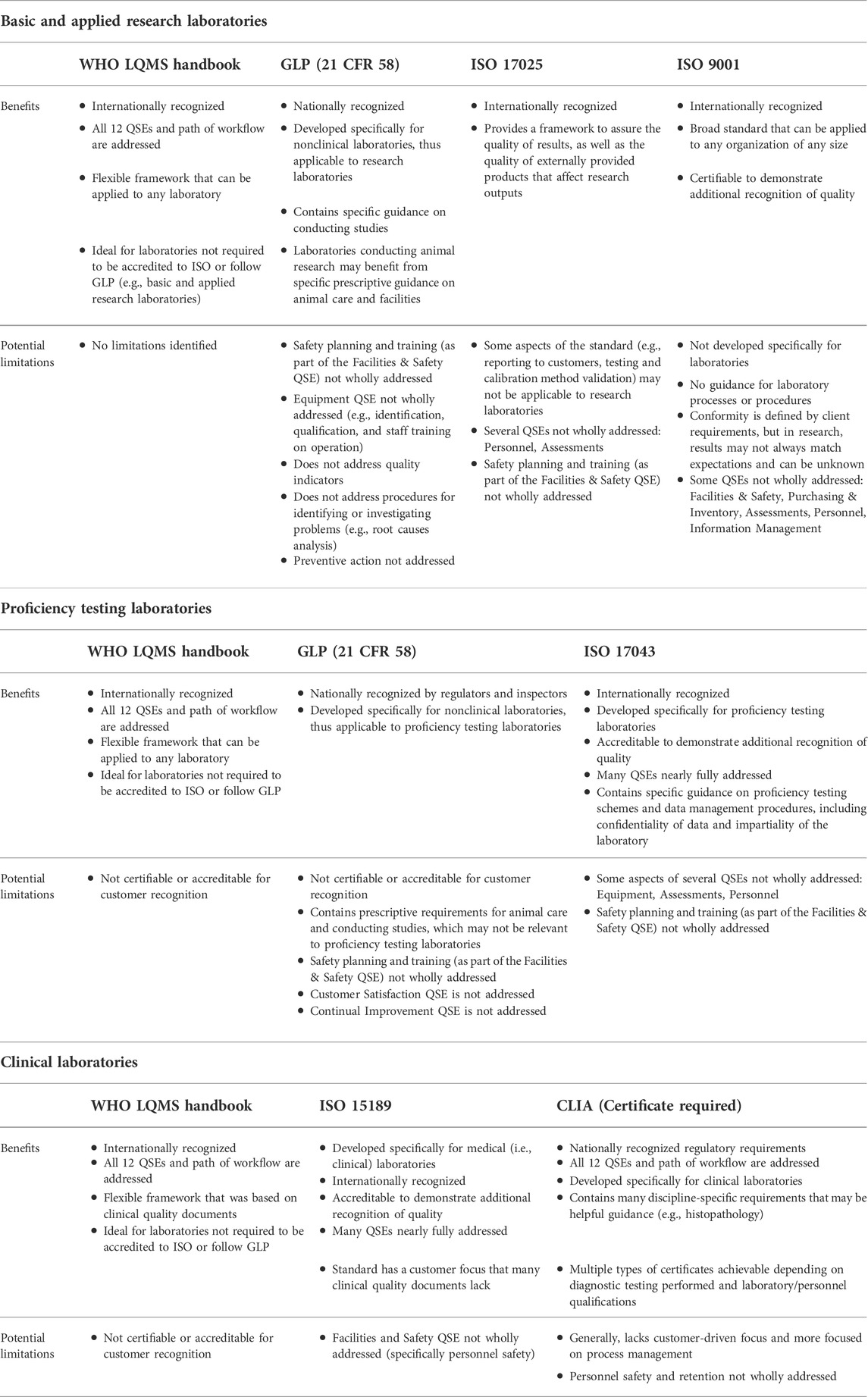
It is critical for a laboratory to consider the type of work conducted and resources available when selecting the most suitable framework. For example, a basic research laboratory conducting studies on plant biology may not benefit from choosing to conform to ISO 17043, a standard that specifies general requirements for proficiency testing laboratories. [International Organization for Standardization, 2010] There are two basic elements for consideration of a framework for suitability: 1) its relevance to the type of work performed by the laboratory, and 2) the sustainability of the framework within the laboratory. Based on analysis of the content, potential intended uses, and approaches of quality management framework documents, Table 2 provides examples of suitable QMS framework documents that could best apply to each major laboratory category, along with benefits and limitations determined based on the crosswalk analysis in Table 1. | |||
[[File:Fig2 Pillai FrontBioengBiotech2022 10.jpg|1145px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="1145px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Table 2.''' Benefits and limitations of select quality management framework documents by major laboratory category.</blockquote> | |||
|- | |||
|} | |||
|} | |||
===Key takeaways=== | |||
From our analysis and crosswalk of quality documents, it was observed that only the [[World Health Organization]]'s (WHO’s) ''Laboratory quality management system: Handbook'' [World Health Organization, 2011], [[ISO 15189]] ''Medical laboratories — Requirements for quality and competence standard'' (International Organization for Standardization, 2012), and CLIA addressed the laboratory’s path of workflow (i.e., total testing process). WHO’s handbook explains this concept and its importance, which is valuable considering that error prevention in each phase helps to ensure the quality of outputs. While not written for laboratories, the ISO 9001 standard for a QMS [International Organization for Standardization, 2015] was included in our analysis due to its broad flexibility and ability to integrate with a laboratory’s institutional processes. One notable finding in the crosswalk is that many of the international quality standards, the [[Food and Drug Administration]] (FDA) regulation document “Good Laboratory Practice for Nonclinical Laboratory Studies” (21 CFR Part 58; hereafter referred to as “GLP”) [FDA, 1978], and CLIA do not fully address personnel safety as a component of the "Facilities and Safety" QSE, but rather focus on facility maintenance and contamination control. (This could be due to the fact that other national regulatory bodies address environmental and occupational safety, such as the Occupational Safety and Health Administration [OSHA], Nuclear Regulatory Commission [NRC], and [[United States Environmental Protection Agency|Environmental Protection Agency]] [EPA].) Additionally, it was observed that purchasing and inventory guidance was also limited when compared to WHO’s laboratory QMS framework. For example, while GLP includes requirements for reagent labeling, GLP does not have a requirement for inspection upon receipt or conducting routine inventory. Another key finding is that the clinical quality documents and GLP do not have as strong of a customer focus as the international quality standards; the focus in these documents is geared towards analytical or study conduct and process management. Establishing procedures on customer service or adhering to the customer service aspects of a suitable ISO standard may be advisable in any environment in order to ensure expectations are continually met. Ultimately, the findings in Tables 1 and 2 demonstrate that a holistic laboratory QMS framework, complemented with guidance from additional quality documents, can benefit many laboratories that aim to address all 12 QSEs. | |||
==References== | ==References== | ||
| Line 49: | Line 106: | ||
==Notes== | ==Notes== | ||
This presentation is faithful to the original, with only a few minor changes to spelling, grammar, and presentation. The PMCID and DOI were also added when they were missing from the original reference. | This presentation is faithful to the original, with only a few minor changes to spelling, grammar, and presentation. The PMCID and DOI were also added when they were missing from the original reference. The original does not provide a citation for the claims concerning the 12 quality system essentials (QSEs); this version adds a citation. The original's singular footnote was turned into a citation for this version. | ||
<!--Place all category tags here--> | <!--Place all category tags here--> | ||
Revision as of 23:52, 17 December 2022
| Full article title | Practical considerations for laboratories: Implementing a holistic quality management system |
|---|---|
| Journal | Frontiers in Bioengineering and Biotechnology |
| Author(s) | Pillai, Segaran; Calvery, Jennifer; Fox, Elizabeth |
| Author affiliation(s) | Food and Drug Administration, Booz Allen Hamilton |
| Primary contact | Email: Segaran dot Pillai at fda dot hhs dot gov |
| Editors | Quemada, H. |
| Year published | 2022 |
| Volume and issue | 10 |
| Article # | 1040103 |
| DOI | 10.3389/fbioe.2022.1040103 |
| ISSN | 2296-4185 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.frontiersin.org/articles/10.3389/fbioe.2022.1040103/full |
| Download | https://www.frontiersin.org/articles/10.3389/fbioe.2022.1040103/pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
A quality management system (QMS) is an essential element for the effective operation of research, clinical, testing, or production/manufacturing laboratories. As technology continues to rapidly advance and new challenges arise, laboratories worldwide have responded with innovation and process changes to meet the continued demand. It is critical for laboratories to maintain a robust QMS that accommodates laboratory activities (e.g., basic and applied research; regulatory, clinical, or proficiency testing), records management, and a path for continuous improvement to ensure that results and data are reliable, accurate, timely, and reproducible. A robust, suitable QMS provides a framework to address gaps and risks throughout the laboratory's workflow that could potentially lead to a critical error, thus compromising the integrity and credibility of the institution. While there are many QMS frameworks (e.g., a model such as a consensus standard, guideline, or regulation) that may apply to laboratories, ensuring that the appropriate framework is adopted based on the type of work performed and that key implementation steps are taken is important for the long-term success of the QMS and for the advancement of science. Ultimately, a well-considered QMS ensures accurate results, efficient operations, and increased credibility, enabling protection of public health and safety. Herein, we explore QMS framework options for each identified laboratory category and discuss prerequisite considerations for implementation. An analysis of various frameworks’ principles and conformity requirements demonstrates the extent to which they address basic components of effective laboratory operations and guides optimal implementation to yield a holistic, sustainable framework that addresses the laboratory’s needs and the type of work being performed.
Keywords: quality, quality management system, laboratory, implementation, framework, total quality, reproducibility
Introduction
In order for laboratories to support the public health mission and better address emerging public health challenges, the need for a quality management system (QMS) that facilitates risk-based thinking and enhances assurance of data quality becomes more critical. With the worldwide shock of the coronavirus disease 2019 (COVID-19) outbreak, organizations have been forced to pivot and innovate to address new issues. The rapid response across industries towards COVID-19 and other crises illustrates business resilience and emphasizes the importance of quality management in the laboratory and its organizational culture. However, incidents stemming from gaps in quality management and affecting public health continue to occur. Laboratory errors have a reported frequency of 0.012%–0.6% of all test results, which in turn has huge impact on diagnosis, as 60 to 70% of all diagnoses are made based upon laboratory tests. [Agarwal, 2014] In addition, there is evidence of an observed data reproducibility crisis in the research community that would benefit from attention and improvement. A recent Nature survey of 1,576 researchers found that 52% of those surveyed agree that there is a crisis of reproducibility; the same survey found that over 70% have failed to reproduce another scientist’s data. [Baker, 2016]
A QMS has numerous benefits that contribute to managing risks in the laboratory, including errors, and mitigating reproducibility crisis concerns. One major benefit is enabling the laboratory to better identify, assess, and address risks faced in the laboratory at all levels of the organization. Poor data quality is one such risk. Some standards, such as the International Organization for Standardization's (ISO's) ISO 31000, are completely dedicated to risk management [International Organization for Standardization, 2018], while other more holistic quality management standards, guidelines, and regulations—such as ISO/IEC 17025—incorporate the concept of risk-based thinking throughout their respective frameworks. [International Organization for Standardization, 2017] In turn, risk management and other aspects of quality management can be built into all three phases of the workflow path (commonly referred to as the "total testing process") of a laboratory: pre-analytical, analytical, and post-analytical. [World Health Organization, 2011]
The pre-analytic phase of a laboratory’s workflow encompasses the activities that are completed prior to operational testing and research (e.g., sample collection and transport). These activities are performed to prepare and support the laboratory in its operations, research, and services. The analytic phase of a laboratory’s workflow encompasses the activities that are completed within the laboratory during operational testing and research. These activities are performed to directly execute the laboratory’s operations, research, and services (e.g., sample testing, experimental studies). Finally, the post-analytic phase of a laboratory’s workflow encompasses the activities that are completed after operational testing and research (e.g., reporting). These activities assure that processes are conducted in a manner that ensures compliance, accuracy, customer satisfaction, and continual improvement.
Each of these three phases must be addressed when implementing a QMS. While someone may suppose that the highest risk of error would occur in the analytic phase, there is now incontrovertible evidence that the majority of laboratory errors occur in the pre-analytical phase (61.9%–68.2%), which are followed by mistakes in the post-analytical phase (18.5%–23.1%) and analytical phase (13.3%–15%). [Mrazek et al., 2020] Within the path of workflow, laboratory processes and procedures can be organized into 12 management areas, known as quality system essentials (QSEs); the 12 QSEs are globally recognized principles that address all aspects of a QMS and cover the entire path of workflow of a laboratory. [CLSI clsi.org/standards-development/quality-system-essentials/] When all of the laboratory procedures and processes are organized into an understandable and workable structure, the opportunity to ensure that all are appropriately managed is increased. [World Health Organization, 2011] Moreover, as safety is a key QSE [CLSI clsi.org/standards-development/quality-system-essentials/], laboratories such as those handling pathogens and other biosafety risks have the opportunity to evaluate the effectiveness of their biosafety procedures and implement improvements (e.g., take measures to reduce the risk of contamination). An integrated, robust QMS can help the laboratory cope with uncertainties that naturally occur in all laboratory environments.
In his 1993 book Preventing Chaos in a Crisis: Strategies for Prevention, Control, and Damage Limitation, Patrick Lagadec emphasizes that the response to an emergency cannot be developed unless the institution has prepared to adapt [Lagadec, 1993]:
...the ability to deal with a crisis situation is largely dependent on the structures that have been developed before chaos arrives. The event can in some ways be considered as an abrupt and brutal audit: at a moment’s notice, everything that was left unprepared becomes a complex problem, and every weakness comes rushing to the forefront.
Given the naturally complex operations of the modern laboratory, minimizing preventable problems and applying lessons learned should be a primary goal.
Defining major laboratory categories
Laboratories are distributed widely across the United States and deal with diverse and unique challenges as a result of differing locations, type of work being performed, and federal, state and local regulations. The role of modern laboratories is quickly evolving as the delivery of health care undergoes drastic changes in the face of unprecedented challenges. With drastic changes comes the need to effectively manage them to ensure continued quality of products and services. It will be critical for a laboratory, belonging to any of the below categories, to reevaluate its alignment with the organization’s strategic direction and quality policy, continually assess risk and impact of changes, carefully define organizational roles and responsibilities, and foster a strong culture of quality and safety.
Testing laboratories (including regulatory laboratories)
Testing laboratories conduct conformance testing to ensure the safety, efficacy, and security of a vast array of materials, including, but not limited to, human and veterinary drugs, biological products, medical devices, food, water, cosmetics, radiation-emitting products, and tobacco products. Testing laboratories are driven by and generate results to support statutory obligations to protect the public and minimize risk associated with such products or materials.
Product development and manufacturing laboratories
Product development and manufacturing laboratories are a subset of testing laboratories that conduct routine quality control analysis, as well as pre-market research and development analysis during design phases, in order to ensure conformity of manufactured products (e.g., test reagents, devices to support analysis, testing or diagnostics) against a set of criteria or attributes selected and agreed upon by the institution. Product development and manufacturing laboratories are driven largely by regulatory requirements, or customer requirements and satisfaction.
Basic and applied research laboratories
Research laboratories can be divided into basic and applied research units. While there is some natural overlap between the two, there are key differences. Basic research is conducted to seek understanding and form answers to scientific questions. According to the Association of American Medical Colleges (AAMC), basic science research—often called fundamental or bench research—provides the foundation of knowledge for the applied science that follows. [AAMC www.aamc.org/what-we-do/mission-areas/medical-research/basic-science] Applied research laboratories conduct studies that are designed to solve practical problems. While considered separate entities from regulatory laboratories, basic and applied research laboratories conduct their work to support and inform the understanding of science, (such as pathogenesis, side effects, efficacy of medical countermeasures, development and evaluation of technology and devices, etc.) and to support regulatory requirements.
Proficiency testing laboratories
Proficiency testing laboratories assess the performance of individual laboratories responsible for specific tests or measurements generating data for regulatory consideration. Results are used to evaluate laboratories’ continuing performance, an important aspect of laboratory quality management and ensuring data integrity.
Clinical laboratories
Clinical laboratories conduct a variety of testing on clinical specimens to evaluate patients’ health. Their work aids in the diagnosis, treatment, and prevention of disease. The Centers for Medicare and Medicaid Services (CMS) regulates clinical laboratory testing performed on human specimens in the United States through the Clinical Laboratory Improvement Amendments (CLIA). [Center for Medicare and Medicaid Services, 2020]
Recommendation: Identify the correct laboratory quality management system framework for the type of laboratory work conducted
Laboratories should strive to gain a thorough understanding of their current state, goals, and objectives prior to selecting and implementing a QMS framework. Determining the major category of the laboratory will help to narrow the focus of the target state and identify if there is any overlapping of categorization that can be addressed in different ways. Capturing the current needs of the laboratory, customers/stakeholders and their requirements, and applicable rules and regulations for which compliance is required will help the laboratory to understand the current state in order to identify the most suitable standard or framework that will govern their QMS and serve as the benchmark for the target state. Once the laboratory's requirements are understood, an appropriate framework or standard for the QMS can then be selected. The subsequent sections provide an analysis of well-known QMS frameworks that can serve to guide laboratories in selecting the most appropriate model for implementation, beginning with a framework crosswalk analysis.
Crosswalk of the quality system essentials and quality management standard requirements
Table 1 presents a crosswalk analysis of well-known quality management framework documents against the framework of 12 QSEs. These QSEs cover all basic elements of a laboratory's QMS. Each standard was reviewed against the QSE framework, aligning a section (e.g., clause, subclause, subpart) to the applicable QSE in order to estimate the coverage of laboratory quality for the given quality management standard.
|
Analysis of quality management standards by laboratory category
It is critical for a laboratory to consider the type of work conducted and resources available when selecting the most suitable framework. For example, a basic research laboratory conducting studies on plant biology may not benefit from choosing to conform to ISO 17043, a standard that specifies general requirements for proficiency testing laboratories. [International Organization for Standardization, 2010] There are two basic elements for consideration of a framework for suitability: 1) its relevance to the type of work performed by the laboratory, and 2) the sustainability of the framework within the laboratory. Based on analysis of the content, potential intended uses, and approaches of quality management framework documents, Table 2 provides examples of suitable QMS framework documents that could best apply to each major laboratory category, along with benefits and limitations determined based on the crosswalk analysis in Table 1.
|
Key takeaways
From our analysis and crosswalk of quality documents, it was observed that only the World Health Organization's (WHO’s) Laboratory quality management system: Handbook [World Health Organization, 2011], ISO 15189 Medical laboratories — Requirements for quality and competence standard (International Organization for Standardization, 2012), and CLIA addressed the laboratory’s path of workflow (i.e., total testing process). WHO’s handbook explains this concept and its importance, which is valuable considering that error prevention in each phase helps to ensure the quality of outputs. While not written for laboratories, the ISO 9001 standard for a QMS [International Organization for Standardization, 2015] was included in our analysis due to its broad flexibility and ability to integrate with a laboratory’s institutional processes. One notable finding in the crosswalk is that many of the international quality standards, the Food and Drug Administration (FDA) regulation document “Good Laboratory Practice for Nonclinical Laboratory Studies” (21 CFR Part 58; hereafter referred to as “GLP”) [FDA, 1978], and CLIA do not fully address personnel safety as a component of the "Facilities and Safety" QSE, but rather focus on facility maintenance and contamination control. (This could be due to the fact that other national regulatory bodies address environmental and occupational safety, such as the Occupational Safety and Health Administration [OSHA], Nuclear Regulatory Commission [NRC], and Environmental Protection Agency [EPA].) Additionally, it was observed that purchasing and inventory guidance was also limited when compared to WHO’s laboratory QMS framework. For example, while GLP includes requirements for reagent labeling, GLP does not have a requirement for inspection upon receipt or conducting routine inventory. Another key finding is that the clinical quality documents and GLP do not have as strong of a customer focus as the international quality standards; the focus in these documents is geared towards analytical or study conduct and process management. Establishing procedures on customer service or adhering to the customer service aspects of a suitable ISO standard may be advisable in any environment in order to ensure expectations are continually met. Ultimately, the findings in Tables 1 and 2 demonstrate that a holistic laboratory QMS framework, complemented with guidance from additional quality documents, can benefit many laboratories that aim to address all 12 QSEs.
References
Notes
This presentation is faithful to the original, with only a few minor changes to spelling, grammar, and presentation. The PMCID and DOI were also added when they were missing from the original reference. The original does not provide a citation for the claims concerning the 12 quality system essentials (QSEs); this version adds a citation. The original's singular footnote was turned into a citation for this version.